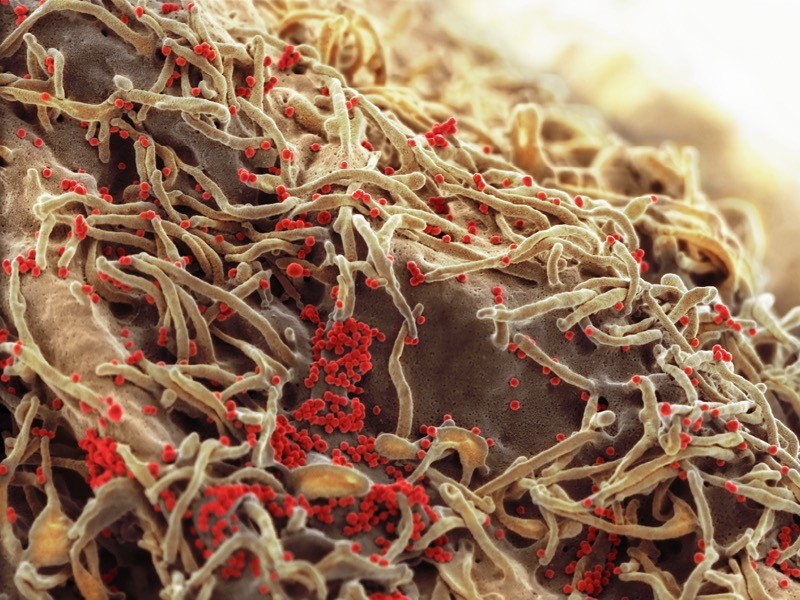
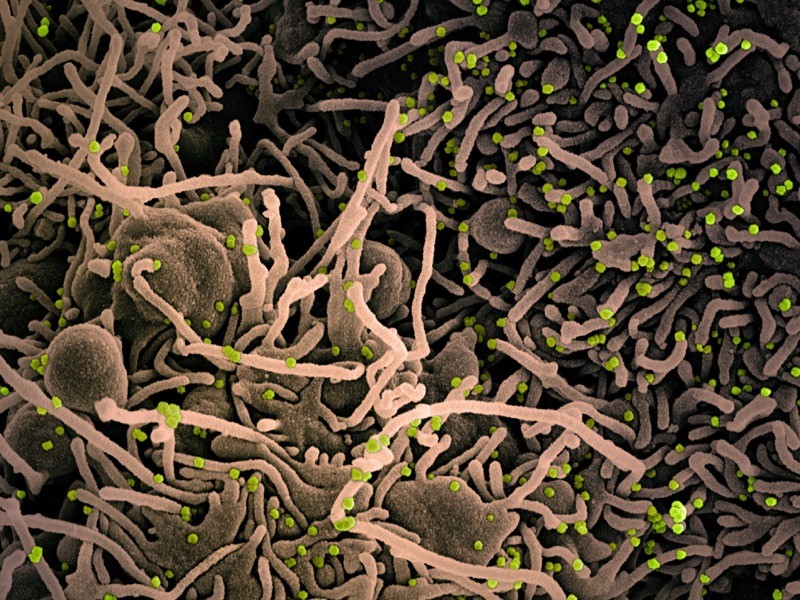
12 March — A small piece of land yields bats with a trove of new coronaviruses
Bats in the province of Yunnan in southern China have yielded yet more coronaviruses closely related to the pandemic virus.
Weifeng Shi at the Shandong First Medical University & Shandong Academy of Medical Sciences in Taian, China, and his colleagues studied 302 samples of faeces and urine and 109 mouth swabs taken from 342 live bats between May 2019 and November 2020 (H. Zhou et al. Preprint at bioRxiv https://doi.org/gh73mk; 2021). The researchers trapped and released all the bats, which represented nearly two dozen species, in an area covering roughly 1,100 hectares — less than one-tenth the size of San Francisco, California.
From the samples, the team sequenced 24 coronavirus genomes, of which 4 were new viruses closely related to SARS-CoV-2. One of the viruses isolated from a Rhinolophus pusillus bat shared 94.5% of its genome with the pandemic virus, making it the second-closest known relative to SARS-CoV-2. The closest known relative is a coronavirus called RATG13, which shares 96% of its genome with SARS-CoV-2 and was isolated from a Rhinolophus affinis bat in Yunnan in 2013.
The results suggest that viruses closely related to SARS-CoV-2 continue to circulate in bats and are highly prevalent in some regions, the researchers say. The findings have not yet been peer reviewed.
11 March — Viral variant causes a more deadly form of COVID
People infected with the coronavirus variant called B.1.1.7 are at a higher risk of dying than are people infected with other circulating variants, regardless of their age, sex and pre-existing health problems.
Daniel Grint at the London School of Hygiene & Tropical Medicine and his colleagues studied the health records of 184,786 people in England who tested positive for SARS-CoV-2 between 16 November 2020 and 11 January 2021. Of these individuals, 867 died by 5 February 2021 (D. Grint et al. Preprint at medRxiv https://doi.org/fzwq; 2021).
The researchers found that for three people who died within a month of testing positive for a previously circulating viral variant, some five died after testing positive for B.1.1.7. The risk of death increases with age and the presence of pre-existing health problems, and men are at higher risk of dying than women.
First detected in the United Kingdom, B.1.1.7 is now the dominant variant there and is spreading widely across Europe. Without control measures and vaccines, the variant could cause a more deadly pandemic than previously circulating versions of the virus, the researchers say.
10 March — A worrisome coronavirus variant brings hope for better vaccines
People infected with a fast-spreading coronavirus variant mount an immune response that can fend off multiple SARS-CoV-2 strains.
Scientists first identified the SARS-CoV-2 variant called B.1.351 in South Africa in late 2020. They have since linked it to reinfections and found hints that several vaccines are less effective against it than against SARS-CoV-2 variants circulating earlier in the pandemic.
Penny Moore at the National Institute for Communicable Diseases in Johannesburg, South Africa, and her colleagues assessed the antibody responses mounted by 89 people who were infected with B.1.351 and were admitted to hospital (T. Moyo-Gwete et al. Preprint at bioRxiv https://doi.org/fzq5; 2021). The team found that these participants’ antibody levels were similar to those in people infected with earlier strains.
The team then pitted antibodies from people infected with B.1.351 against a form of HIV modified to use the coronavirus spike protein to infect cells. The antibodies were able to inactivate viruses incorporating the form of spike protein found in B.1.351, earlier strains, and an emerging variant identified in Brazil called P.1.
The results suggest that vaccines based on B.1.351’s genetic sequence might protect people from multiple strains of the coronavirus, the authors say. The findings have not yet been peer reviewed.
5 March — T cells might provide rescue from rampant coronavirus variants
Emerging coronavirus variants do not seem to elude important immune-system players called T cells, laboratory studies suggest.
Some recently discovered SARS-CoV-2 variants can partially evade antibodies generated in response to vaccination and previous infection,raising fears that vaccines will be less effective against the variants than against the original strain of the virus. Alessandro Sette and Alba Grifoni at the La Jolla Institute for Immunology in California and their colleagues looked at whether these variants’ mutations might also help them to evade T cells — a component of the immune system that is particularly important for reducing the severity of infectious diseases (A. Tarke et al. Preprint at bioRxiv https://doi.org/gh6tkp; 2021).
The team collected T cells from volunteers who had either recovered from infection with the ancestral SARS-CoV-2 strain or had received an mRNA coronavirus vaccine. The researchers then tested the cells’ ability to recognize protein snippets from four emerging variants, including the B.1.351 variant first identified in South Africa.
Most of the volunteers’ T cells recognized all four variants, thanks to viral protein snippets that were unaffected by the variants’ mutations. The results suggest that T cells could target these variants.
4 March — A new viral variant hits a COVID-ravaged city
A coronavirus variant detected in the Brazilian city of Manaus might be driving reinfections and the city’s second wave of COVID-19.
During the first wave of the pandemic, Manaus experienced one of the world’s highest infection rates: an estimated two-thirds of residents were infected by October 2020, leading some researchers to predict that population-wide immunity might cause new infections to tail off. But in January 2021, researchers identified a novel coronavirus variant, called P.1, during a period of rising hospitalizations in the city and linked the variant to a few cases of reinfection.
To characterize the variant further, Nuno Faria, at Imperial College London, and his colleagues analysed viral genomes collected from 184 human samples in Manaus between November and December (N. R. Faria et al. Preprint at https://go.nature.com/3sor3jj; 2021). The variant harbours 17 mutations that alter SARS-CoV-2 proteins. Among the alterations are changes in the SARS-CoV-2 spike protein that have been previously linked to increased transmission and immune evasion.
By modelling the spread of P.1 and its possible effects during Manaus’ second wave, the researchers estimated that the variant was 1.4–2.2 times more transmissible than other lineages and that it was able to evade some of the immunity conferred by previous infections. The findings have not yet been peer reviewed.
3 March — Kids in the classroom could mean COVID at home
Living with children who are attending school in person raises an adult’s risk of developing COVID-19 symptoms, but only if schools don’t implement appropriate control measures, according to a large online survey in the United States.
Justin Lessler at Johns Hopkins University in Baltimore, Maryland, and his colleagues analysed responses to a Facebook survey completed in late 2020 and early 2021 by more than half a million people living with school-aged children; half of these respondents had children attending school in person, either full-time or part-time (J. Lessler et al. Preprint at medRxiv; https://doi.org/fxv6; 2021).
The researchers found that adults living with kids — especially high-school students — who went into school were more likely to report COVID-19 symptoms or test positive for SARS-CoV-2. But the researchers also found that schools could eliminate that risk entirely by implementing at least seven mitigation measures from a list that included requiring students and teachers to wear masks, preventing parents from entering schools and increasing the spacing between desks. The findings have not yet been peer reviewed.
2 March — Just one dose of vaccine protects against silent COVID infection
Asymptomatic coronavirus infections were four times less frequent in health-care workers who had received a single dose of a prominent COVID-19 vaccine than in their unvaccinated counterparts.
Michael Weekes at the University of Cambridge, UK, and his colleagues analysed the results of almost 8,900 SARS-CoV-2 tests taken by UK health-care workers without symptoms of COVID-19 (M. Weekes et al. Preprint at Authorea https://doi.org/fxkd; 2021). Study participants who were tested at least 12 days after receiving one dose of the vaccine developed by Pfizer of New York City and BioNTech of Mainz, Germany, had an infection rate of only 0.2%. By contrast, unvaccinated participants had an infection rate of 0.8%.
The team also noted that participants who showed evidence of SARS-CoV-2 infection well after vaccination tended to have lower levels of the coronavirus in their bodies than did those who were infected and unvaccinated, although the result did not reach statistical significance. If corroborated, this would suggest that the few vaccinated health-care workers who do have an asymptomatic infection are less likely to infect other people than are unvaccinated workers who become infected.
The findings have not yet been peer reviewed.
26 February — A COVID vaccine passes a real-world test with flying colours
The two-shot Pfizer vaccine is highly effective at preventing severe COVID-19, according to an analysis of more than one million people in Israel.
Ran Balicer at Clalit Health Services in Tel Aviv, Israel, and his colleagues matched 596,618 people vaccinated as part of a nationwide campaign with an unvaccinated ‘twin’ of the same age, sex, ethnicity and neighbourhood of residence. The pairs also had a matching number of medical conditions and shared other characteristics (N. Dagan et al. N. Engl. J. Med. https://doi.org/fw7w; 2021).
The researchers found that, at 7 days or more after the second shot, Pfizer’s vaccine was 94% effective at preventing COVID-19 and 92% effective against severe disease. The results were consistent across all age groups, including in people aged 70 and older. The results were strikingly close to efficacy estimates from clinical trials, despite being based on jabs administered in less stringently controlled settings and more diverse populations, including people with multiple health problems.
The study also covered a period when the emerging variant called B.1.1.7 was circulating widely in Israel, which suggests that the vaccine is effective at preventing COVID-19 caused by that variant.
25 February — Trial hints that Pfizer vaccine could curb COVID transmission
A leading COVID-19 vaccine is highly effective at preventing SARS-CoV-2 infections, whether they cause symptoms or not — the strongest evidence yet that vaccines could control viral spread.
Large-scale trials of COVID-19 vaccines have focused on assessing their ability to prevent disease, but researchers also want to know whether the vaccines can prevent people from getting infected, even if they show no symptoms. Susan Hopkins at Public Health England in London and her colleagues tracked the effectiveness of the vaccine made by Pfizer and BioNTech in 23,000 UK health-care workers who were already part of a long-term study of SARS-CoV-2 immunity (V. J. Hall et al. Preprint at SSRN https://doi.org/fw7v; 2021). Participants were tested regularly for SARS-CoV-2, regardless of their symptoms.
The vaccine was 70% effective at preventing both symptomatic and asymptomatic infections in the period beginning 3 weeks after the first dose; this grew to 85% shortly after a second dose of the RNA vaccine. This finding is the first evidence that Pfizer’s vaccine might block transmission, the researchers say. The study has not yet been peer reviewed.
23 February — Viral variant is less susceptible to a COVID vaccine’s effects
Coronaviruses engineered to contain mutations from a worrisome variant partially blunt the immune protection offered by a prominent vaccine.
In recent months, studies have raised the possibility that the potent antibodies summoned by vaccines could be less effective against emerging SARS-CoV-2 variants than against older versions of the virus. Most of this work stems from experiments not on SARS-CoV-2, but on viruses such as HIV that have been modified to contain SARS-CoV-2’s signature spike protein.
Pei-Yong Shi at the University of Texas Medical Branch in Galveston and his colleagues engineered several variants of SARS-CoV-2, including one containing the same spike-protein mutations as a troubling variant called B.1.351 (also known as 501Y.V2) that was first identified in South Africa (Y. Liu et al. N. Engl. J. Med. https://doi.org/fwsc; 2021).
The team pitted the B.1.351-like virus against blood serum from people who had received two doses of the vaccine made by Pfizer and BioNTech in Mainz, Germany. Antibodies elicited by the vaccine neutralized the virus only one-third as effectively as they did a strain lacking those mutations.
The researchers traced most of the virus’s evasive ability to a trio of mutations in the portion of the spike protein that SARS-CoV-2 uses to adhere to host cells. However, it is not clear whether these changes make the vaccine less effective at preventing COVID-19.
22 February — A delayed second jab means better protection against COVID
A widely used COVID-19 vaccine is more effective if the second of its two doses is given after a long wait rather than a short one — a finding that supports a decision by UK public-health officials to space out the doses.
The two-dose vaccine developed by the University of Oxford, UK, and pharmaceutical firm AstraZeneca in Cambridge, UK, could be given to more people by lengthening the interval between jabs. To test the efficacy of this strategy, Andrew Pollard at the University of Oxford and his colleagues examined clinical-trial data from more than 17,000 people, half of whom received the vaccine and the other half a placebo (M. Voysey et al. Lancet https://doi.org/fwk7; 2021).
The team found that, for intervals greater than six weeks, the longer the gap between jabs, the better the vaccine protected against COVID-19. It was 55% effective in those who received their second dose less than 6 weeks after their first, and 81% effective in those whose second dose was more than 12 weeks after their first. The team also found that a single dose of the vaccine had an efficacy of 76% for the first 90 days after vaccination.
19 February — Longer infections could fuel a variant’s quick spread
Preliminary findings suggest that B.1.1.7, a SARS-CoV-2 variant first identified in the United Kingdom, might be more transmissible because it spends more time inside its host than earlier variants do.
Previous studies have estimated that B.1.1.7, which is now spreading rapidly in a number of countries, is roughly 50% more contagious than earlier coronavirus variants are. Yonatan Grad at the Harvard T.H. Chan School of Public Health in Boston, Massachusetts, and his colleagues examined the results of daily SARS-CoV-2 tests on 65 people infected with SARS-CoV-2, including 7 infected with B.1.1.7 (S. M. Kissler et al. Preprint at https://nrs.harvard.edu/URN-3:HUL.INSTREPOS:37366884; 2021). The team looked at how long the virus persisted, and the amount of virus present at each time point.
In people infected with B.1.1.7, infections lasted an average of 13.3 days, compared with 8.2 days in people with other variants. There was little difference in the peak concentrations of the virus between the two groups.
These findings hint that B.1.1.7 is more easily transmitted than other variants are because people who catch it are infected for a relatively long time, and can therefore infect a larger number of contacts. This suggests that longer quarantine periods might be warranted for individuals infected with this variant. The findings have not yet been peer reviewed.
16 February — ‘Robin’ leads a flock of new US COVID variants
Seven newly identified coronavirus variants in the United States share a similar mutation, but the significance of this change is not yet clear.
Coronavirus variants emerging in a range of geographical locations seem to share certain mutations — possible evidence that the changes aid transmission. Jeremy Kamil at Louisiana State University Health Sciences Center in Shreveport and his colleagues identified a new variant that they named Robin (E. B. Hodcroft et al. Preprint at medRxiv https://doi.org/fvs4; 2021). It carries a change called Q677P in the SARS-CoV-2 spike protein, which the virus uses to bind to cells.
The lineage was first spotted in October and its prevalence has risen in some parts of the United States. It accounted for 27.8% of sequenced viruses in Louisiana and 11.3% in New Mexico between the start of December 2020 and mid-January 2021. The researchers identified six other variants — also named after birds, including Pelican and Bluebird — in the United States with a mutation at the same spot in the spike protein.
The mutation is located near a portion of the spike protein that must be cut to allow a viral particle to infect a cell, but the researchers say that laboratory studies might be needed to determine what the mutation does. The finding has not yet been peer reviewed.
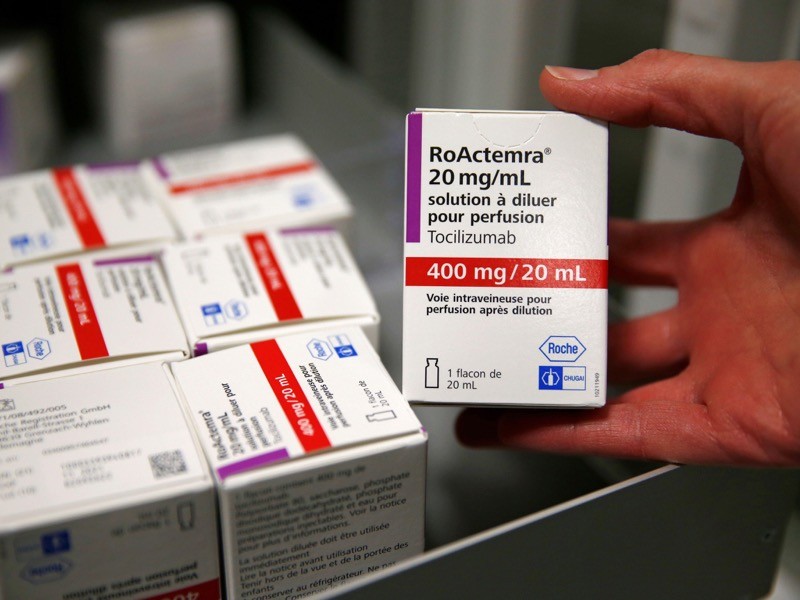
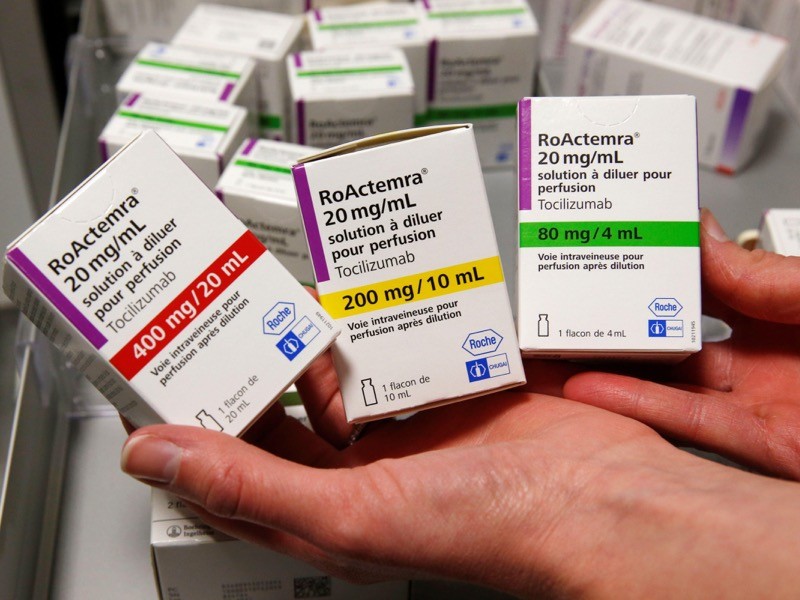
15 February — Drug can be a lifeline for people hospitalized for COVID
An anti-inflammatory drug can save the lives of people hospitalized for COVID-19 whose immune systems have gone into overdrive against the coronavirus. The drug also cuts the need for invasive ventilation, according to a large study.
Many people with severe COVID-19 symptoms show evidence of widespread inflammation. The drug tocilizumab is designed to dampen such an immune response, but previous clinical trials of its benefits in people infected with SARS-CoV-2 have been equivocal.
Peter Horby and Martin Landray at the University of Oxford, UK, and their colleagues compared more than 2,000 people treated with tocilizumab with a similar number who did not receive the drug (RECOVERY Collaborative Group. Preprint at medRxiv https://doi.org/fvqj; 2021). Study participants were in hospital and receiving oxygen and had evidence of system-wide inflammation, and nearly all were also taking the steroid dexamethasone.
The authors report that 54% of people who received tocilizumab left hospital within 28 days, compared with 47% of those not taking the drug. An analysis showed that tocilizumab provided benefits on top of those from dexamethasone. The team estimates that roughly half of all people hospitalized with COVID-19 in the United Kingdom would benefit from the drug.
The findings have not yet been peer reviewed.
12 February — Vaccines spur antibody surge against a COVID variant
One shot of either the Moderna or the Pfizer vaccine provokes a strong immune response against an emerging variant of SARS-CoV-2, according to tests in people who have recovered from COVID-19.
The mRNA vaccines made by Moderna in Cambridge, Massachusetts, and Pfizer in New York City are highly effective at preventing COVID-19 caused by the original form of SARS-CoV-2. Andrew McGuire at the Fred Hutchinson Cancer Research Center in Seattle, Washington, and his colleagues collected blood from ten people who had recovered from COVID-19; they collected additional samples after the study participants had received a single dose of one of the two vaccines (L. Stamatatos et al. Preprint at medRxiv https://doi.org/ft9j; 2021). The researchers then examined the participants’ levels of neutralizing antibodies — which defend cells from infection — against the original version of SAR-CoV-2, which was first detected in Wuhan, China, and against B.1.351, the concerning new variant that was first identified in South Africa.
Before inoculation, nine of the ten individuals had neutralizing antibodies against the original virus, although the levels generated were highly variable. Antibodies from only five people could neutralize B.1.351. Following a single shot of the vaccine, however, participants’ levels of neutralizing antibodies against both forms of the virus increased by approximately 1,000-fold.
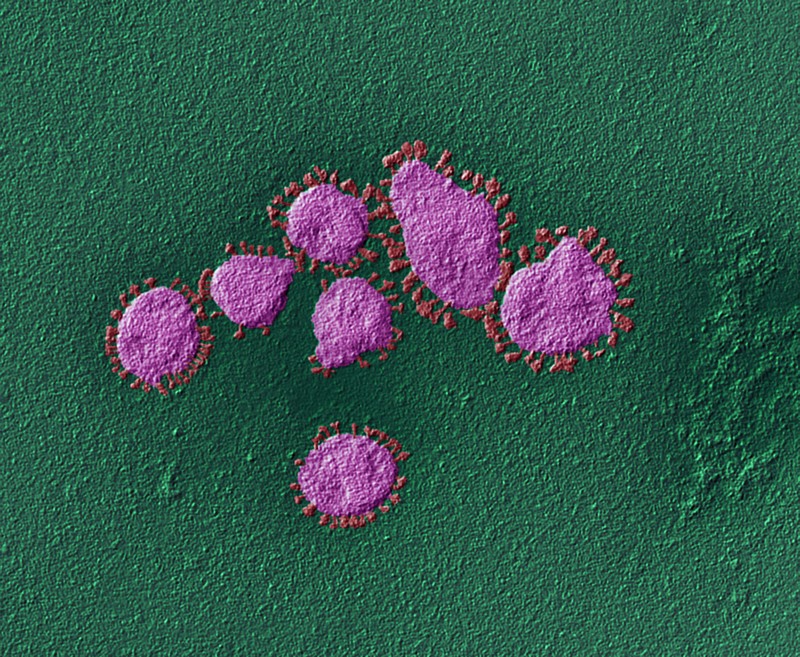
9 February — Nimble coronaviruses could leap straight from bats to humans
Some coronaviruses found in bats could jump directly to people without the need for further evolution in an intermediate animal host.
Victor Garcia at the University of North Carolina at Chapel Hill and his colleagues implanted mice with human lung tissue and infected the tissue with various coronaviruses, including SARS-CoV-2 and two closely related coronaviruses isolated from bats. All of the viruses could efficiently multiply in the lung tissue (A. Wahl et al. Nature https://doi.org/10.1038/s41586-021-03312-w; 2021). The findings suggest that coronaviruses circulating in bats could directly infect people, and have the potential to cause the next pandemic.
The researchers also used the animal model to show that an oral antiviral drug known as EIDD-2801 could significantly reduce infectious particles of SARS-CoV-2 in the lung tissue. They say that the drug, currently in late-stage clinical trials, could be used to prevent disease as well as to treat people within a day or two of exposure to SARS-CoV-2.
8 February — Why it matters that COVID viruses are losing parts of their genome
Again and again, the new coronavirus has sloughed off small chunks of its genome, leading to changes in a viral protein that is frequently targeted by antibodies.
When evolution snips out a stretch of an organism’s genome, the change is called a deletion. Kevin McCarthy and Paul Duprex at the University of Pittsburgh School of Medicine in Pennsylvania and their colleagues searched a database of SARS-CoV-2 genome sequences and identified more than 1,000 viruses with deletions in the genomic region that encodes a protein called spike (K. R. McCarthy et al. Science https://doi.org/10.1126/science.abf6950; 2021). The virus uses the spike protein to invade cells.
Further analysis showed that the deletions tended to crop up at a few distinct sites in the genomic region coding for spike. Some of the deletions have arisen independently multiple times, and some show evidence of spread from one person to another.
A powerful antibody against SARS-CoV-2 could not latch onto spike proteins harbouring some of the deletions that the team identified. But antibody mixtures collected from people who had recovered from COVID-19 could disable viral variants that had deletions.
5 February — One man’s COVID therapy drives worrisome viral mutations
Antibody treatment for COVID-19 seems to have spurred mutations in the SARS-CoV-2 that infected a man with a compromised immune system.
In mid-2020, a man was admitted to hospital with COVID-19. He had been diagnosed with cancer in 2012; the illness and his treatment had probably weakened his immune system. The man’s COVID-19 was treated with two courses of the antiviral drug remdesivir and, later, two courses of convalescent plasma — antibody-laden blood from people who had recovered from COVID-19. He died 102 days after admission.
Ravindra Gupta at the University of Cambridge, UK, and his colleagues analysed viral genomes obtained from the man during his illness (S. A. Kemp et al. Nature https://doi.org/10.1038/s41586-021-03291-y; 2021). The viral populations in his blood changed little after remdesivir treatment. But after each course of convalescent plasma, the samples were dominated by viruses with a particular pair of mutations in the SARS-CoV-2 spike protein, the main target of the immune system.
Experiments showed that one of the mutations weakened the potency of the antibodies in the convalescent plasma, yet also reduced the virus’s infectivity. The second mutation restored infectivity. The potential for viral evolution means that convalescent plasma should be used cautiously when treating people with compromised immunity, the authors say.
4 February — What makes a person with COVID more contagious? Hint: not a cough
The amount of SARS-CoV-2 in a person’s body is a major factor in determining whether they are likely to transmit the virus to others, according to a study of nearly 300 infected people and their close contacts.
Most people with COVID-19 do not give it to anyone else, but some become ‘superspreaders’. To understand why, Michael Marks at the London School of Hygiene & Tropical Medicine and his colleagues monitored 282 people, deemed ‘index cases’, who had recently developed mild symptoms of COVID-19. The team also monitored 753 people who lived with, cared for or otherwise had close contact with the index cases (M. Marks et al. Lancet Infect. Dis. https://doi.org/10.1016/S1473-3099(20)30985-3; 2021).
Only one-third of the index cases transmitted the virus to a close contact. Those with a relatively high ‘viral load’, a measure of the amount of virus in the body, were much more likely to pass on the virus than were those with a low viral load. Index cases were no more likely to transmit the virus if they had a cough than if they didn’t.
The findings suggest that tracing the contacts of people with high viral loads is especially important, the authors say.
2 February — Russia’s Sputnik V vaccine shows high effectiveness
A vaccine that relies on modified cold viruses is more than 91% effective against symptomatic COVID-19, according to interim results of a clinical trial involving nearly 22,000 people.
Pathogens in the adenovirus group typically cause mild illnesses, such as the common cold. The Sputnik V vaccine developed at the Gamaleya National Center of Epidemiology and Microbiology in Moscow consists of two types of adenovirus, each carrying genetic instructions for the spike protein that SARS-CoV-2 uses to latch onto host cells. Use of two viruses could help to increase the immune response to the vaccination.
Denis Logunov at the Gamaleya centre and his colleagues analysed data from roughly 15,000 clinical-trial participants who had received an initial jab containing one type of adenovirus and, 21 days later, a booster jab of the second type of virus. The team also studied 4,900 participants who received two doses of a placebo (D. Y. Logunov et al. Lancet https://doi.org/10.1016/S0140-6736(21)00234-8; 2021).
Starting from 21 days after the first jab, 16 symptomatic cases of COVID-19 — all mild — were recorded in the vaccine group. The placebo group incurred 62 cases, 20 of which were moderate or severe.
Preliminary evidence suggests that the vaccine starts to offer protection 16–18 days after the first dose, but the authors say more research is needed to confirm this early finding.
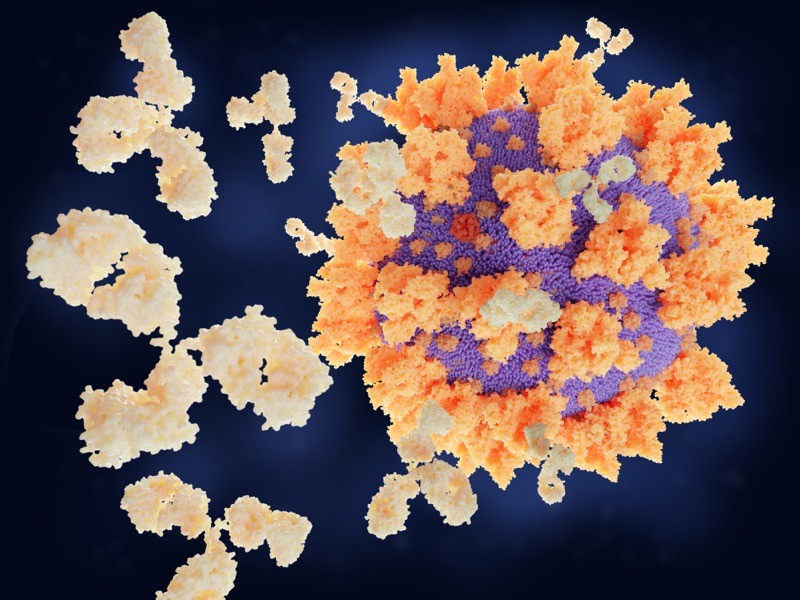
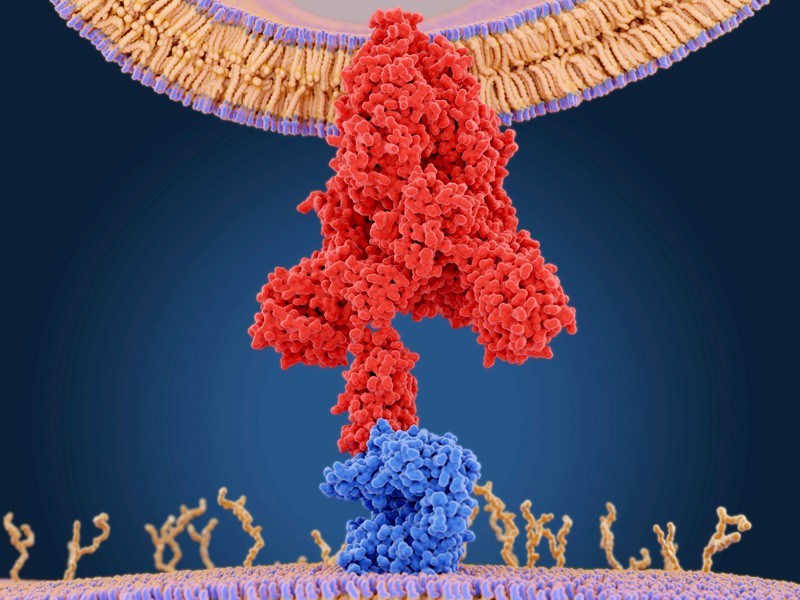
29 January — An antibody that clamps onto the COVID virus’s ‘Achilles heel’
Scientists have engineered an antibody that effectively disables SARS-CoV-2 and closely related coronaviruses.
Laura Walker at the biopharmaceutical company Adimab in Lebanon, New Hampshire, and her colleagues isolated antibodies from the immune cells of a person who had recovered from a 2003 infection with the virus SARS-CoV, which is related to SARS-CoV-2 (C. G. Rappazzo et al. Science https://doi.org/fsbc; 2021). By tinkering with the structure of the antibodies, the researchers created one, called ADG-2, that was particularly effective at disabling SARS-CoV-2 in a lab dish.
The engineered antibody also disabled a variety of related coronaviruses.When given to mice, it stopped SARS-CoV-2 from reproducing in the rodents’ lungs and protected the animals from respiratory disease.
Experiments showed that ADG-2 targets receptors found on the surface of SARS-CoV-2 and a range of similar coronaviruses. The authors dub this receptor the Achilles heel of coronaviruses closely related to SARS-CoV-2, and suggest that this vulnerability could be exploited to make vaccines against emerging coronaviruses.
28 January — The workers who keep us fed face some of the highest COVID risk
The death risk for essential workers in some sectors was 20–40% higher than expected during the first 8 months of the COVID-19 pandemic, according to an analysis of death records in California.
Yea-Hung Chen and his colleagues at the University of California, San Francisco, analysed state data for people aged 18–65 to estimate how many more deaths occurred among working-age adults during the pandemic than would have been expected without the onslaught of SARS-CoV-2 (Y.-H. Chen et al. Preprint at medRxiv https://doi.org/frx9; 2021). The team found that compared with a no-pandemic scenario, deaths were 39% higher for food and agriculture workers, 28% higher for transportation and logistics workers and only 11% higher for non-essential workers.
The workers with the highest COVID-related risk included cooks, bakers, agricultural labourers and people who pack and prepare goods for shipment. Risk also varied by race and ethnicity: compared with the no-pandemic scenario, mortality during the pandemic was 36% higher for people aged 18–65 of Latin American descent overall and 59% higher for food and farm workers in this group.
The authors say essential workers should receive free personal protective equipment and easy access to testing. The findings have not yet been peer reviewed.
26 January — Moderna vaccine vanquishes viral variants
A leading COVID-19 vaccine seems to work against new, rapidly spreading variants of SARS-CoV-2.
Darin Edwards at biotechnology company Moderna in Cambridge, Massachusetts, and his colleagues collected blood samples from 8 people and 24 macaques that had received 2 doses of the company’s vaccine (K. Wu et al. Preprint at bioRxiv https://doi.org/fr2g; 2021). The vaccine instructs the body to make the coronavirus’s spike protein, priming the immune system to produce ‘neutralizing’ antibodies that can prevent cells from being infected. All of the blood samples from vaccinated people and monkeys contained neutralizing antibodies against the virus.
The researchers exposed the blood to viral particles that mimic a range of coronavirus variants, including an emerging form first found in the United Kingdom and another, 501Y.V2, first detected in South Africa. The samples’ neutralizing antibodies were as effective against the variant first found in the United Kingdom as against an older form of the virus, but only about one-fifth to one-tenth as effective at neutralizing 501YV.2. Even so, the antibodies were effective enough to provide protection against both variants, the authors say.
Moderna says it plans to test a booster to enhance immunity to emerging coronavirus variants. The findings have not yet been peer reviewed.
21 January — COVID vaccines might lose potency against new viral variants
Newly emerging, fast-spreading variants of the coronavirus might reduce the protective effects of two leading vaccines.
Michel Nussenzweig at the Rockefeller University in New York City and his colleagues analysed blood from 20 volunteers who received two doses of either the vaccine developed by Moderna or that developed by Pfizer–BioNTech (Z. Wang et al. Preprint at bioRxiv https://doi.org/frdn; 2021). Both vaccines carry RNA instructions that prompt human cells to make the spike protein that the virus uses to infect cells. This causes the body to generate immune molecules called antibodies that recognize the spike protein.
Within 3–14 weeks after the second jab, the study participants developed several types of antibody, including some that can block SARS-CoV-2 from infecting cells. Some of these neutralizing antibodies were as effective against viruses carrying certain mutations in the spike protein as they were against widespread forms of the virus. But some were only one-third as effective at blocking the mutated variants.
Some of the mutations that the team tested have been seen in coronavirus variants that were first identified in the United Kingdom, Brazil and South Africa; at least one of these variants is more easily transmitted than other forms of the virus now in wide circulation.
The findings suggest that vaccine-resistant variants might emerge, meaning that COVID-19 vaccines could need an update. They have not yet been peer reviewed.
Correction 28 January 2021: An earlier version of this article gave an incorrect time window for antibody development after vaccination.
20 January — The unsung viral feature that could lead to more COVID treatments
A usually overlooked region of a key SARS-CoV-2 protein is an important chink in the virus’s armour.
Neutralizing antibodies against SARS-CoV-2 block particles of the virus, which makes them some of the body’s most potent weapons against the new pathogen. Most of the neutralizing antibodies that researchers have studied target a region of the virus’s spike protein called the receptor-binding domain (RBD). But previous studies have also identified neutralizing antibodies that act against other portions of spike — particularly a region called the N-terminal domain (NTD).
David Veesler at the University of Washington in Seattle and his colleagues analysed the blood of people who had recovered from COVID-19, and identified 41 antibodies that recognize the NTD (M. McCallum et al. Preprint at bioRxiv https://doi.org/fq92; 2021). Some proved to be as potent at blocking infection as were antibodies that recognize the RBD. Hamsters treated with one of the strongest NTD-targeting antibodies were protected from SARS-CoV-2 infection.
Worryingly, the authors found that variants of the coronavirus first identified in the United Kingdom and South Africa carry mutations that might weaken the effects of some NTD antibodies. The findings have not yet been peer reviewed.
19 January — Immune cells ‘remember’ COVID for at least half a year
The immune system remembers how to make antibodies that can fend off the new coronavirus for at least six months after the initial infection.
Levels of antibodies to SARS-CoV-2 often wane in the months following infection, which has raised concerns that immunity to the virus declines rapidly. Michel Nussenzweig at the Rockefeller University in New York City and his colleagues collected blood samples from 87 people about 1 month and 6 months after they were infected with the virus (C. Gaebler et al. Nature https://doi.org/fq6k; 2021). The team monitored participants’ levels of antibodies and memory B cells, immune cells that would stimulate the production of antibodies against the virus if the study participants were reinfected.
The team found that levels of antibodies against the coronavirus’s spike protein declined over six months. But participants’ levels of memory B cells specific for making antibodies against the spike protein remained constant. The researchers sampled the intestines of 14 participants 4 months after infection and found that half had persistent SARS-CoV-2 protein or RNA, potentially providing a continued source of stimulation to the immune system.
15 January — Two anti-inflammatory drugs prevent COVID deaths
Two drugs that dampen the body’s immune response can save the lives of people with severe COVID-19.
Some people gravely ill with COVID-19 have tissue damage inflicted by their own immune response, and also show increased activity of immune-system molecules and cells regulated by a protein called IL-6. To study the effect of quashing IL-6 activity, Anthony Gordon of Imperial College London and his colleagues tested the drugs tocilizumab and sarilumab, which block the protein that immune cells use to detect IL-6 (A. C. Gordon et al. Preprint at medRxiv https://doi.org/fqgf; 2021).
The team gave the drugs to 803 adults with COVID-19 who were in intensive care and receiving organ support, such as ventilation or high-flow oxygen. Of these participants, 353 received tocilizumab, 48 received sarilumab and 402 received neither. The drug treatment reduced the death rate — from nearly 36% in the control group to 28% among those who received tocilizumab and 22% for sarilumab.
The results have not yet been peer reviewed.
14 January — A potential COVID vaccine causes a durable immune response
A candidate vaccine spurs both younger and older people to make antibodies against the coronavirus SARS-CoV-2, according to early trial results.
The vaccine, which is under development by Johnson & Johnson, headquartered in New Brunswick, New Jersey, uses a harmless virus to inject cells with the instructions for making the SARS-CoV-2 spike protein. Hanneke Schuitemaker at Janssen Vaccines and Prevention in Leiden, the Netherlands, and her colleagues tested the vaccine’s safety and immune-stimulating properties in more than 800 people aged 18 years and up (J. Sadoff et al. N. Engl. J. Med. https://doi.org/fqnt; 2021).
Almost 100% of study participants aged 18–55 years had developed potent antibodies against the virus 57 days after receiving a single low dose of the jab. In a separate arm of the trial, the same regimen triggered the development of antibodies in 96% of participants aged 65 years and older 29 days after vaccination. Side effects were largely mild or moderate, and antibodies persisted until at least 71 days after inoculation.
The results of larger efficacy trials of the vaccine are pending.
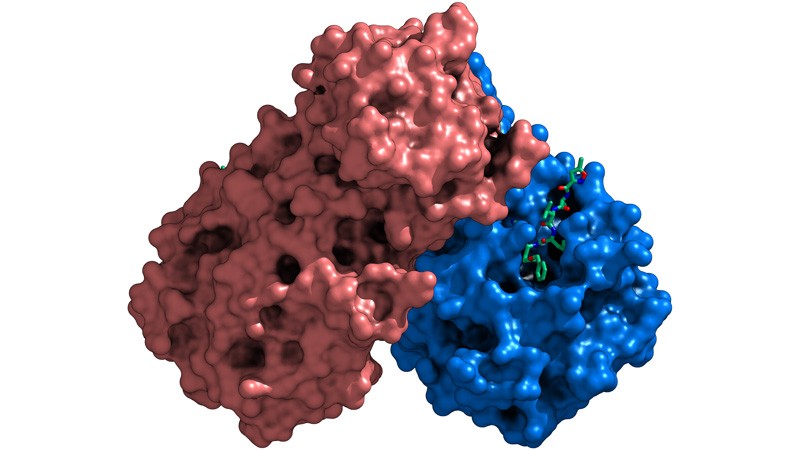
13 January — A mutation undercuts the immune response to the COVID virus
A handful of mutations to SARS-CoV-2 can help it to escape the immune response mounted by a subset of infected people.
Researchers have identified thousands of mutations in SARS-CoV-2 samples, but the vast majority are unlikely to have much effect on the virus’s biology. To identify potentially important mutations, Jesse Bloom at the Fred Hutchinson Cancer Research Center in Seattle, Washington, and his colleagues studied antibodies against SARS-CoV-2 isolated from the blood serum of people who had recovered from COVID-19 (A. J. Greaney et al. Preprint at bioRxiv https://doi.org/ghr85d; 2021).
The team tested the antibodies’ response to samples of the virus’s spike protein. Each sample protein carried different versions of a region called the receptor binding domain (RBD), which recognizes host cells and is a major target for antibodies.
Of thousands of RBD mutations tested, only a few reduced the antibodies’ ability to bind tightly to the spike protein — a change that might also indicate a reduction in the antibodies’ ability to disable the virus.
But the effects varied substantially between people. The most consequential mutations, at a location called E484, caused a steep drop in the potency of some individuals’ antibodies. Coronavirus variants identified in South Africa and Brazil carry a mutation at the same spot.
The findings have not yet been peer reviewed.
12 January — Immune cells gone wild are tied to COVID lung damage
Some of the severe respiratory symptoms of COVID-19 seem to result from the activity of specific immune cells, which can cause long-term inflammation of the lungs.
Alexander Misharin at Northwestern University in Evanston, Illinois, and his colleagues examined fluid from the lungs of 88 people with severe pneumonia caused by SARS-CoV-2 infection (R. A. Grant et al. Nature https://doi.org/fqds; 2021). Most of these individuals had high numbers of a certain type of T cell, a class of immune cells, in their lungs. The researchers also found that nearly 70% of alveolar macrophages, a type of immune cell that is located in the tiny air sacs of the lungs, contained SARS-CoV-2. The cells harbouring the virus showed relatively high expression of genes involved in inflammation.
The findings suggest that, once the virus reaches the lungs, it can infect macrophages, which respond by producing inflammatory molecules that attract T cells. T cells, in turn, produce a protein that stimulates macrophages to make more inflammatory molecules. This persistent lung inflammation could lead to some of the life-threatening consequences of SARS-CoV-2 infection.
11 January — Traitorous antibodies are linked to COVID death
Antibodies normally attack pathogens, but, sometimes, rogue antibodies instead besiege bodily components such as immune cells. Now, a new study adds to the growing body of research tying these ‘autoantibodies’ to poor outcomes in people with COVID-19.
Ana Rodriguez and David Lee at the NYU Grossman School of Medicine in New York City and their colleagues studied autoantibody levels in blood serum collected from 86 people who required hospitalization for COVID-19. The researchers were particularly interested in autoantibodies against the protein annexin A2, which helps to stabilize cell-membrane structure. It also plays a part in ensuring the integrity of tiny blood vessels in the lungs. Blocking annexin A2 leads to lung injury, a hallmark of COVID-19.
The scientists found that the level of anti-annexin A2 antibodies was, on average, higher in the individuals who eventually died of COVID-19 than in those who survived — a difference that was statistically significant (M. Zuniga et al. Preprint at medRxiv https://doi.org/fqdd; 2021).
More research is necessary to establish a clear causal link between the virus SARS-CoV-2 and autoantibodies against annexin A2, which are relatively rare. The findings have not yet been peer reviewed.
8 January — Quick treatment with antibody-laden blood cuts risk of severe COVID
A clinical trial in older adults with COVID-19 shows that an early dose of blood plasma from recovered people helps to prevent the progression to severe disease.
The plasma of people who have recovered from COVID-19 contains antibodies against SARS-CoV-2. But treatment with such plasma has had mixed results, and some scientists have suggested that plasma needs to be given early in the disease course to be effective. Fernando Polack at Fundación INFANT in Buenos Aires and his colleagues conducted a rigorous clinical trial to assess the effect of treatment with plasma within 72 hours of symptom onset. Participants included people over the age of 75 and those between 65 and 74 with at least one pre-existing condition such as diabetes (R. Libster et al. N. Engl. J. Med. https://doi.org/10.1056/NEJMoa2033700; 2021).
Severe COVID-19 developed in 16% of the 80 study participants who received plasma and 31% of the 80 participants in the placebo group. The team found that donor plasma containing higher concentrations of antibodies against SARS-CoV-2 was associated with a greater reduction in the risk of developing severe disease — providing evidence that the antibodies themselves are responsible for the therapeutic effect.
7 January — Evidence grows of a new coronavirus variant’s swift spread
Two independent analyses have found that a new SARS-CoV-2 variant overtaking the United Kingdom is indeed more transmissible than other forms of the virus.
Eric Volz and Neil Ferguson at Imperial College London and their colleagues examined nearly 2,000 genomes of the variant, which has been labelled variant of concern 202012/01. The genomes were collected in the United Kingdom between October and early December 2020. The team also analysed the results of roughly 275,000 UK COVID-19 tests administered in late 2020 (E. Volz et al. Preprint at medRxiv https://doi.org/ghrqv8; 2021).
Estimating the variant’s frequency over time, the authors concluded that it is roughly 50% more transmissible than other variants. The authors also found that a UK lockdown in November curbed COVID-19 cases caused by most viral variants — but cases linked to the new variant rose. The findings have not yet been peer reviewed.
A separate team also used genomic and other data to analyse the variant’s spread in the last few months of 2020. Nicholas Davies and his colleagues at the London School of Hygiene and Tropical Medicine estimated that the new variant is 56% more transmissible than other variants (N. Davies et al. Preprint at medRxiv https://doi.org/fp3v; 2020). The authors found no evidence that the variant of concern causes more severe COVID-19 than other variants. The findings are now under peer review.
6 January — A less-sensitive COVID test could help to curb outbreaks
Rapid COVID-19 tests that trade away a degree of reliability for speed could prove a valuable public-health tool in communities that are hit hard by the disease.
In one week, Diane Havlir at the University of California, San Francisco, and her colleagues tested about 3,300 people in the city for SARS-CoV-2 (G. Pilarowski et al. Clin. Inf. Dis. https://doi.org/fpzv; 2020). All the study volunteers had two tests: the gold-standard PCR test, which typically returns results in two to four days in the United States; and a rapid test that detects viral proteins called antigens and returned results in roughly one hour. The rapid test, BinaxNOW, is made by Abbott Laboratories in Abbott Park, Illinois.
The rapid test detected 89% of the 237 people who tested positive with PCR — and it detected all of those who had high levels of the virus. Within two hours of a positive rapid-test result, participants received a phone call advising them to isolate themselves. This swift response meant people were less likely to spread the infection than they might have been had they waited for a PCR result. Approximately 1% of the positive rapid antigen tests were not confirmed by PCR — meaning they were wrong.
Study author Havlir disclosed that she receives non-financial support from Abbott that is not related to the paper.
4 January ― A vaccine works quickly to ward off COVID-19
An RNA-based vaccine recently approved by US regulators can provide protection against COVID-19 within two weeks of the first dose, according to the results of a large clinical trial.
On 18 December, the US Food and Drug Administration granted an emergency-use authorization to a vaccine made by Moderna in Cambridge, Massachusetts. Shortly thereafter, Lindsey Baden at Brigham and Women’s Hospital in Boston, Massachusetts, Hana El Sahly at Baylor College of Medicine in Houston, Texas, and their colleagues published the results of a vaccine trial that enrolled more than 30,000 volunteers. Half received two doses of a placebo and half received two doses of the vaccine, 28 days apart (L. R. Baden et al. N. Engl. J. Med. https://doi.org/ghrg8m; 2020).
The vaccine was 94% effective at preventing symptomatic COVID-19, and preliminary analysis hints that just one dose of the vaccine might also provide some defence against asymptomatic disease, the authors write. All 30 trial participants who developed severe COVID-19 were in the placebo arm.
About half of volunteers who received the vaccine experienced side effects such as headaches after their second dose. But serious side effects were rare and occurred as frequently in the placebo group as in the vaccinated group.
21 December — How 90% of French COVID cases evaded detection
In the weeks after France ended its first lockdown, nine residents with COVID-19 symptoms went undetected for every person confirmed to have the disease — despite a nationwide surveillance programme.
France reopened in May but adopted a strategy of testing, contact tracing and case isolation to keep the coronavirus in check. To assess the results, Vittoria Colizza at the Pierre Louis Institute of Epidemiology and Public Health in Paris and her colleagues modelled COVID-19 transmission in France between mid-May and late June. They found that the national testing campaign missed some 90,000 people with COVID-19 who showed symptoms at a time when infections in the country were declining (G. Pullano et al. Nature https://doi.org/fn9k; 2020).
The findings show that a low rate of positive test results does not always equate to a high rate of detected cases. The results also suggest that many people with symptoms of COVID-19 did not seek medical advice or testing.
Countries need to implement more aggressive and efficient testing of people with suspected infections if surveillance is to be a useful tool for fighting the pandemic, the researchers say.
18 December — Stay-at-home orders have limited value for curbing COVID
An analysis of COVID-19 data from 41 countries has identified 3 measures that each substantially cut viral transmission: school and university closures, restricting gatherings to no more than 10 people and shutting businesses. But adding stay-at-home orders to those actions brought only marginal benefit.
Questions linger about the relative effectiveness of specific measures to reduce the spread of SARS-CoV-2. To pinpoint the most useful, Jan Brauner at the University of Oxford, UK, and his colleagues modelled the number of new SARS-CoV-2 infections in 41 countries between 22 January and either 30 May or the first easing of restrictions (J. M. Brauner et al. Science https://doi.org/ghp2p7; 2020). The team also examined when each country implemented seven common anti-transmission measures.
By combining the two data sets, the researchers found that closing schools and universities had a “large effect” in dampening viral spread. Most countries closed schools and universities in quick succession, making it impossible for the team to disentangle the effects of each type of closure.
In countries that closed schools and businesses and restricted gatherings, a stay-at-home order did little more to reduce transmission, the authors found.
16 December — Self-sabotaging antibodies are linked to severe COVID
Antibodies usually fight off infection, but occasionally the immune system makes some that erroneously attack the body’s own organs and even the immune system itself. New results show that these ‘autoantibodies’ might explain why some people have a severe reaction to infection with SARS-CoV-2.
Akiko Iwasaki and Aaron Ring at the Yale School of Medicine in New Haven, Connecticut, and their colleagues studied 194 people with COVID-19 and found that the most seriously ill had high levels of autoantibody activity (E. Y. Wang et al. Preprint at medRxiv https://doi.org/fnkt; 2020). Some of the autoantibodies attacked the body’s immune cells, hampering the ability to fight off infection. Others attacked the central nervous system, the heart, the liver or connective tissue.
No single autoantibody was common enough to be used to distinguish people with COVID-19 from uninfected people. The authors say the diversity of autoantibodies could explain the various disease states that follow COVID-19.
14 December — A drug duo that helps people with severe COVID
A combination of the drugs baricitinib and remdesivir shaved one day off the recovery of people hospitalized with COVID-19.
The US National Institutes of Health recommends remdesivir as a treatment for some people with COVID-19. But questions linger about remdesivir’s effectiveness, and the World Health Organization cautions against its use.
To test it as part of a combination therapy, Andre Kalil at the University of Nebraska Medical Center in Omaha and his colleagues gave remdesivir and the anti-inflammatory drug baricitinib to roughly 500 people hospitalized with moderate or severe COVID-19 (A. C. Kalil et al. N. Engl. J. Med. https://doi.org/ghpbd2; 2020). Some 500 people in a control group received remdesivir and a placebo. The team monitored how long it took participants to recover enough to go without sustained medical care.
Those who took both drugs had a median time to recovery of seven days, compared with eight days for those who took only remdesivir. But for people who were on the edge of requiring invasive ventilation, median recovery time fell from 18 days on remdesivir alone to 10 days on both drugs.
11 December — How to save the most lives when a COVID vaccine is scarce
Front-line health-care workers will probably be the first to get COVID-19 vaccines, but who should be next in the queue when supplies are limited? Models suggest that it should be elderly people.
Kate Bubar and Daniel Larremore at the University of Colorado Boulder and their colleagues modelled the effects of rolling out a vaccine if various age groups are given priority (K. M. Bubar et al. Preprint at medRxiv https://doi.org/ghj6xw; 2020). The researchers also examined the influence of the rate of viral spread in the population, the speed of vaccine delivery and the effectiveness of the protection offered by the vaccine.
The team found that in most scenarios, giving the jabs to people older than 60 before those in other age groups saved the greatest number of lives. But to prevent as many people as possible from getting infected, countries should prioritize younger age groups, according to the analysis.
Targeting people who have not been infected with SARS-CoV-2 to receive the vaccine might cut deaths and infections in hard-hit regions further, the researchers say. This could be achieved by testing for antibodies against SARS-CoV-2, which indicates a history of recent infection. The findings have not yet been peer reviewed.
8 December — A coronavirus vaccine shows lasting benefit
People given a front-runner COVID-19 vaccine still had high levels of potent antibodies against the coronavirus four months after their first jab.
Biotech firm Moderna in Cambridge, Massachusetts, has reported that its vaccine is more than 94% effective at preventing COVID-19. To gauge whether this protection lasts, Alicia Widge at the US National Institute of Allergy and Infectious Diseases in Bethesda, Maryland, and her colleagues analysed blood from 34 study volunteers who received two doses of the vaccine one month apart (A. T. Widge et al. N. Engl. J. Med. https://doi.org/ghnhnv; 2020).
The volunteers’ levels of antibodies that latch on to a key SARS-CoV-2 protein peaked 1–2 weeks after the second jab and fell only slightly in the subsequent 2.5 months. Four months after the first jab, their blood still contained ‘neutralizing’ antibodies that disable the virus, and none of the participants had experienced any serious vaccine-related side effects.
The results show that the vaccine could provide a “durable” antibody response, the authors write.
7 December — Just a pinch of antibodies can protect against COVID
Low levels of antibodies to the new coronavirus might be sufficient to protect against COVID-19, according to a study of infected monkeys. The study also found that immune cells called T cells contribute to immunity to the virus, particularly when antibody levels are low.
There is no easy way to predict which aspects of an immune response will provide protection against an infectious disease. Dan Barouch at Harvard Medical School in Boston, Massachusetts, and his colleagues sought to understand which immune elements defend against COVID-19 using rhesus macaques (Macaca multatta).
The team collected antibodies from macaques that were recovering from SARS-CoV-2 infection and gave the antibodies to uninfected macaques (K. McMahan et al. Nature https://doi.org/fmjk; 2020). The antibodies protected the recipient animals from infection and boosted a host of immune responses, including the activation of antibody-dependent natural killer cells. Higher doses of antibodies conferred greater protection than did lower doses.
When the researchers reduced the recovering macaques’ levels of CD8+ T cells, the animals’ immunity to re-infection fell. This suggests that these cells also contribute to coronavirus immunity.
4 December — Smell tests could sniff out rising COVID case counts
A fast, cheap test of a person’s ability to smell could help to stop COVID-19 outbreaks, according to models.
Previous studies have reported that more than three-quarters of people infected with SARS-CoV-2 lose some or all of their sense of smell — a statistic that holds true even for those who do not feel ill. This distinctive symptom prompted Roy Parker at the University of Colorado Boulder and his colleagues to model whether mass testing for loss of smell could help to quash an epidemic (D. B. Larremore et al. Preprint at medRxiv https://doi.org/fmbb; 2020).
The team’s simulations showed that a smell test administered every three days could prevent a spike in infections in a population of 20,000 people, assuming that at least 50% of infected people experienced a detectable loss of smell. The tests would also be effective for surveillance before mass events such as aeroplane flights, the modelling showed.
Study author Daniel Larremore disclosed that he advises test company Darwin Biosciences; author Derek Toomre disclosed that he is a founder of smell-test company u-Smell-it. The findings have not yet been peer reviewed.
3 December — The mutations that let the coronavirus give antibodies the slip
Scientists have identified a SARS-CoV-2 mutation that allows the virus to escape recognition by several antibodies manufactured as COVID-19 treatments.
The designer therapies called monoclonal antibodies are modelled on naturally occurring immune molecules. Jesse Bloom at the Fred Hutchinson Cancer Research Center in Seattle, Washington, and his colleagues mapped every possible SARS-CoV-2 mutation that could prevent binding by three monoclonal antibodies: one manufactured by Eli Lilly in Indianapolis, Indiana, and the two in a ‘cocktail’ made by Regeneron in Tarrytown, New York (T. N. Starr et al. Preprint at bioRxiv https://doi.org/fk6h; 2020).
The mutations affect a protein segment called the receptor-binding domain, which the virus uses to bind to and enter cells. The researchers found one mutation that caused the virus to escape recognition by Regeneron’s antibody cocktail, and a few others that helped it to escape one of the three antibodies.
Few of these mutations are circulating widely in infected people. But one is prevalent in Europe, and another has been detected in the Netherlands and Denmark, where it has been found in SARS-CoV-2 samples taken from mink and people working at mink farms. The findings have not yet been peer reviewed.
2 December ― Why a sensitive COVID test can yield false negatives
The gold-standard method for diagnosing COVID-19 is the polymerase chain reaction (PCR) test, which detects the coronavirus’s genetic material in a nose or throat swab. Now, a survey of more than 15,000 people has singled out the people most likely to receive false negatives on the test.
Caitlin Dugdale at Massachusetts General Hospital in Boston and her colleagues looked at PCR test results from about 15,000 people who showed COVID-19 symptoms or were thought for other reasons to be infected with SARS-CoV-2 (C. M. Dugdale et al. Open Forum Infect. Dis. https://doi.org/fkw4; 2020). Nearly 2,700 individuals tested negative and had a second PCR test done within 2 weeks.
Among those who received a second test, 60 — or 2.2% — tested positive. Of these, 60% had their initial test either one day or less before symptom onset or more than 7 days after it, suggesting that the PCR test is most likely to yield a false negative in people tested early or late in the course of infection.
People with COVID-19 symptoms who test negative should be retested, especially in areas where the virus is widespread, the researchers say.
1 December — To avoid COVID, beware your nearest and dearest
A far-reaching study of SARS-CoV-2 transmission in China’s Hunan Province found that the encounters that were most likely to spread the coronavirus were those between members of the same household.
Kaiyuan Sun at the National Institutes of Health in Bethesda, Maryland, Hongjie Yu at Fudan University in Shanghai, China, and their colleagues analysed data from 1,178 people in Hunan who were infected with SARS-CoV-2 and more than 15,000 close contacts of the infected people (K. Sun et al. Science https://doi.org/fkwm; 2020). The team found that contacts between people who live together posed the greatest risk of transmission, followed by contacts between members of an extended family. The transmission risk was lower still for social contacts and community encounters, such as those on public transport. Every extra day of contact raised transmission risk by 10%, the team found.
The analysis suggests that Hunan’s lockdown actually increased the risk of viral spread within households, whose members spent more time than normal at home together during lockdown. But social and community transmission fell during the same period.
20 November — Immune responses to coronavirus persist beyond 6 months
The immune system’s memory of the new coronavirus lingers for at least six months in most people.
Sporadic accounts of coronavirus reinfection and reports of rapidly declining antibody levels have raised concerns that immunity to SARS-CoV-2 could dwindle within weeks of recovery from infection. Shane Crotty at the La Jolla Institute for Immunology in California and his colleagues analysed markers of the immune response in blood samples from 185 people who had a range of COVID-19 symptoms; 41 study participants were followed for at least 6 months (J. M. Dan et al. Preprint at bioRxiv https://doi.org/ghkc5k; 2020).
The team found that participants’ immune responses varied widely. But several components of immune memory of SARS-CoV-2 tended to persist for at least 6 months. Among the persistent immune defenders were memory B cells, which jump-start antibody production when a pathogen is re-encountered, and two important classes of T cell: memory CD4+ and memory CD8+ T cells. The results have not yet been peer-reviewed.
19 November — The coronavirus mutates rapidly as it races through mink farms
The coronavirus is adapting to its mink hosts, suggests a genetic analysis of farmed animals infected with SARS-CoV-2.
Outbreaks of coronavirus have been reported on farms breeding mink (Neovison vison and Mustela lutreola) across Europe and the United States since April. François Balloux at University College London and his colleagues studied 239 viral genomes isolated from farmed animals in the Netherlands and Denmark (L. van Dorp et al. Preprint at bioRxiv https://doi.org/fjj6; 2020).
The team identified at least seven separate instances in which the virus had jumped from people infected with SARS-CoV-2 to mink. The researchers also found 23 mutations that had arisen independently at least twice, suggesting that the virus was rapidly adapting to its new host.
Some of these frequent mutations appeared in regions of the genome that encode the spike protein that coronaviruses use to infect cells. But researchers say there is no evidence that these changes in mink will affect SARS-CoV-2’s ability to spread in people if it jumps back to humans. The findings have not yet been peer reviewed.
17 November — Colds caused by coronaviruses might not ward off COVID
Although SARS-CoV-2 can be deadly, it has mild-mannered cousins called seasonal coronaviruses that are among the causes of the common cold. Some scientists have suggested that people might be shielded from SARS-CoV-2 infection if they have recently been infected by a seasonal coronavirus.
Scott Hensley at the University of Pennsylvania in Philadelphia and his colleagues examined blood samples collected before the pandemic from some 500 people (E. M. Anderson et al. Preprint at medRxiv https://doi.org/fh2n; 2020). The team found that all of the study participants had pre-pandemic antibodies that could recognize the seasonal coronavirus OC43. One-quarter of participants also had antibodies that could recognize SARS-CoV-2, which probably developed in response to infection with a common-cold coronavirus.
Half of the participants went on to catch SARS-CoV-2. Individuals who became infected and those who didn’t had similar levels of antibodies recognizing SARS-CoV-2. This suggests that neither those antibodies nor those that recognize OC43 offer protection against infection, the authors say.
17 November — Quick COVID tests catch the people who are most infectious
Rapid antigen tests for the coronavirus are faster, cheaper and more user-friendly than standard diagnostic assays. An assessment now shows that some antigen tests — but not all — can tell with high accuracy who is likely to be most infectious.
Antigen-based assays detect specific proteins, or antigens, on the surface of SARS-CoV-2 particles. Christian Drosten at the Charité — University Hospital Berlin and his colleagues analysed the performance of seven commercially available rapid antigen tests. The researchers applied the tests to a range of samples, including dozens of swabs from people who had already tested positive for SARS-CoV-2 or for other respiratory viruses using the gold-standard polymerase chain reaction test (V. M. Corman et al. Preprint at medRxiv https://doi.org/ghj3wt; 2020).
The five most sensitive antigen assays detected the presence of SARS-CoV-2 on 95% of the test runs for samples with concentrations of viral genetic material that ranged between the equivalent of 3.4 million and 74 million copies per millilitre of swab. Such high viral levels are observed during the first week of symptoms, when people are likely to spread the virus to others. The findings have not yet been peer reviewed.
13 November — The coronavirus can mutate swiftly in one person’s body
The new coronavirus resurged again and again in the body of an infected man, eventually killing him while showing evidence of fast-paced evolution.
Manuela Cernadas and Jonathan Li at Brigham and Women’s Hospital in Boston, Massachusetts, and their colleagues followed the course of COVID-19 in a 45-year-old man with a long-standing autoimmune disorder, who was on a medication regimen that included powerful immunosuppressants (B. Choi et al. N. Engl. J. Med. https://doi.org/fhv8; 2020). Roughly 40 days after the man first tested positive for SARS-CoV-2, follow-up tests indicated that the virus was dwindling — but it surged back, despite antiviral treatment.
The man’s infection subsided and then returned twice more before he died, five months after his first COVID-19 diagnosis. Genomic analysis showed that the man had not been infected multiple times. Instead, the virus had lingered and quickly mutated in his body.
12 November — The mystery of one African nation’s low COVID death toll
One of the first large SARS-CoV-2 antibody studies in Africa suggests that by mid-2020, the virus had infected 4% of people in Kenya — a surprisingly high figure in view of Kenya’s small number of COVID deaths.
The presence of antibodies against SARS-CoV-2 indicates a history of infection with the virus. Sophie Uyoga at the KEMRI-Wellcome Trust Research Programme in Kifili, Kenya, and her colleagues searched for such antibodies in samples of blood donated in Kenya between late April and mid-June (S. Uyoga et al. Science https://doi.org/fhsx; 2020). Based on those samples, the researchers estimate that 4.3% of Kenya’s people had a history of SARS-CoV-2 infection.
The team’s estimate of antibody prevalence in Kenya is similar to an earlier estimate for the level in Spain. But Spain had lost more than 28,000 people to COVID-19 by early July, whereas Kenya had lost 341 by the end of the same month. The authors write that the “sharp contrast” between Kenya’s antibody prevalence and its COVID-19 deaths hints that the coronavirus’s effects are dampened in Africa.
11 November — A coronavirus mutation could weaken antibodies’ power
A widespread variant of the new coronavirus has the potential to evade the immune response that some people mount after infection.
Since the start of the pandemic, researchers have identified thousands of viral mutations in the genomes of SARS-CoV-2 samples taken from infected people. David Robertson at the University of Glasgow, UK, Gyorgy Snell at Vir Biotechnology in San Francisco, California, and their colleagues examined a mutation called N439K in a protein that the virus uses to invade cells (E. C. Thomson et al. Preprint at bioRxiv https://doi.org/fhnp; 2020).
The mutation affects the protein’s receptor binding domain, which it uses to recognize host cells, and which is a key target of antibodies against the virus. The mutation has emerged independently at least twice and has been identified in 12 countries.
In laboratory experiments, the researchers found that the mutation could hinder the activity of potent neutralizing antibodies that block the virus. Among the neutralizing antibodies that the mutation obstructed were those in the blood of people who had recovered from COVID-19, as well as some manufactured ‘monoclonal antibodies’ that are being developed into treatments. The findings have not yet been peer reviewed.
9 November — Uninfected children have antibodies to the coronavirus
Scientists have found antibodies that recognize SARS-CoV-2 in the blood of people who have never caught the virus. Children are particularly likely to harbour such antibodies, which might explain why most infected children have either mild illness or none at all.
It has been unclear whether previous infection with one of the ‘seasonal’ coronaviruses — which cause the common cold — wards off SARS-CoV-2 or its severe symptoms. George Kassiotis at the Francis Crick Institute in London and his colleagues analysed blood samples from both adults and children who had not been infected with the new virus (K. W. Ng et al. Science https://doi.org/fg9k; 2020). The samples were collected either before the pandemic began or just as the virus began its global march.
The team found that roughly 5% of 302 uninfected adult participants had antibodies that recognize SARS-CoV-2. So did more than 60% of uninfected participants aged 6 to 16 — the age group in which antibodies to seasonal coronaviruses are most common. Most blood samples from uninfected people who had antibodies to SARS-CoV-2 blocked the new coronavirus from infecting cells in lab dishes.
6 November — A vaccine that mimics the coronavirus prompts potent antibodies
A COVID-19 vaccine candidate made of tiny artificial particles could be more powerful than other leading varieties at triggering a protective immune response.
David Veesler and Neil King at the University of Washington in Seattle and their colleagues designed microscopic ball-shaped particles that mimic the structure of a virus (A. C. Walls et al. Cell https://doi.org/fg6r; 2020). The researchers fused 60 copies of SARS-CoV-2’s spike protein — the part of the virus that allows it to infect human cells — to the outside of each of these ‘nanoparticles’.
When the team injected mice with the nanoparticle vaccine, the animals produced virus-blocking antibodies at levels comparable to or greater than those produced by people who had recovered from COVID-19. Mice that received the vaccine produced about ten times more of these antibodies than did rodents vaccinated only with the spike protein, on which many COVID-19 vaccine candidates rely.
The vaccine also appears to produce a strong response from special immune cells that help to mount a fast defence after infection with SARS-CoV-2.
4 November — Many surfaces carry coronavirus RNA — but not much of it
Swabbing of bank machines, shop-door handles and other frequently touched surfaces in a US city revealed that 8% of samples were positive for SARS-CoV-2 genetic material, but that material was present in small amounts.
Amy Pickering at Tufts University in Medford, Massachusetts, and her colleagues repeatedly sampled 33 surfaces in public places in Somerville, Massachusetts (A. P. Harvey et al. Preprint at medRxiv https://doi.org/fgx9; 2020). The handles of a rubbish bin and a liquor store were the most frequently riddled with coronavirus RNA. All samples showed only “low-level” contamination, and the infection risk from touching one of the contaminated surfaces is low, the researchers say.
The team found that the percentage of positive samples in one postal district peaked roughly 7 days before a spike in COVID-19 cases in the same district. Sampling of heavily touched surfaces might provide a warning of a surge of infections, the authors write. The findings have not yet been peer reviewed.
2 November — The coronavirus’s spread in households is fast and often silent
The new coronavirus spreads more efficiently in US homes than previous research suggested,sometimes without any symptoms to warn of its transmission, according to intensive monitoring of more than 100 US households.
Melissa Rolfes at the Centers for Disease Control and Prevention in Atlanta, Georgia, and her colleagues recruited 101 US residents who had tested positive for SARS-CoV-2 and had recently developed COVID-19 symptoms (C. G. Grijalva et al. Morb. Mortal. Wkly Rep. https://doi.org/fgx3; 2020). For at least a week after enrolment, the researchers gathered daily coronavirus test results from 191 people who lived with the infected people.
For a conservative estimate of disease spread, the researchers excluded contacts who tested positive when their household signed up for the study. All the same, 35% of the remaining participants eventually tested positive — almost double one previous estimate. Fewer than one half of household contacts who became infected showed symptoms when they first tested positive, and 75% tested positive five days or less after the first infected person in their home began feeling ill.
2 November — How 45 countries rank on coronavirus infections
A country’s tally of COVID-19 deaths among those aged under 65 can be used to reveal the total number of people who have been infected.
Megan O’Driscoll at the University of Cambridge, UK, and her colleagues compared data on COVID-19 deaths across 45 countries (M. O’Driscoll et al. Nature https://doi.org/fgts; 2020). The researchers found that among people younger than 65, the risk of dying of COVID-19 increased with age in a pattern that was consistent across all countries.
The team also compiled statistics from 22 studies in 16 countries on the percentage of people who had SARS-CoV-2 antibodies, which indicate that they have previously been exposed to the virus. This helped the authors to estimate the infection fatality rate, which is the proportion of people who die after being infected with SARS-CoV-2, for the 45 study countries.
The researchers combined the infection fatality rates and the under-65 death statistics to estimate that by the beginning of September, some 5% of the 3.4 billion people in the 45 countries studied had been infected with SARS-CoV-2. South Korea had the lowest infection rate, at just 0.06%, and Peru had the highest with 62%.
This method could be used to estimate how many people have been infected in areas that cannot carry out large antibody surveys, the researchers say.
30 October — Coronavirus-fighting antibodies linger for months
The body’s antibody defence against the coronavirus remains strong for at least three months after infection, according to a study of more than 100 people who mostly had mild to moderate COVID-19.
Ania Wajnberg at the Icahn School of Medicine at Mount Sinai in New York City and her colleagues analysed blood samples from more than 30,000 people who had been infected with SARS-CoV-2 (A. Wajnberg et al. Science https://doi.org/fgfs; 2020). More than 90% of the infected people had moderate or high levels of antibodies in their blood, and experiments showed that these antibodies could block the virus from infecting cells.
The team also re-tested a subset of 121 individuals at two later dates and found that their antibody levels were stable for at least three months. Five months after symptom onset, the individuals’ antibodies showed only a modest decline . The authors say that it is “very likely” that the antibodies shield people from re-infection.
28 October — A fur-farm animal can spread the coronavirus
The small fox-like animals called raccoon dogs (Nyctereutes procyonoides) can be infected with SARS-CoV-2, and can spread it among themselves.
Conrad Freuling at the Friedrich Loeffler Institute in Greifswald–Isle of Riems, Germany, and his colleagues deliberately infected nine raccoon dogs with the new coronavirus (C. M. Freuling et al. Emerg. Infect. Dis. https://doi.org/ffzf; 2020). Six began shedding the virus from their noses and throats several days later. When three uninfected animals were put in cages next to the infected animals, two got infected. None of the animals became visibly sick, but some were slightly lethargic.
These findings suggest that SARS-CoV-2 could spread undetected in fur farms in China, where more than 14 million raccoon dogs live in captivity. The coronavirus that caused the pandemic of severe acute respiratory syndrome in 2002–2004 was also isolated in raccoon dogs, and could have first jumped to people from the canids.
27 October — Basketball stars score with coronavirus insights
Professional basketball players in the United States have helped to provide details of a poorly understood phase of SARS-CoV-2’s life cycle: its behaviour in the bodies of newly infected people.
After a four-month hiatus, US basketball games resumed in July. Before and after play restarted, athletes and staff members were repeatedly checked for SARS-CoV-2 with a version of the highly sensitive polymerase chain reaction method, which can be used to assess a person’s viral levels. The intensive testing offered a rare chance to monitor viral levels in infected people who had not yet developed symptoms, and in those who never felt ill.
Stephen Kissler at the Harvard T.H. Chan School of Public Health in Boston, Massachusetts, and his colleagues analysed test results from 68 people involved in the season (S. M. Kissler et al. Preprint at medRxiv https://doi.org/ffxk; 2020). Study participants’ viral levels peaked about three days after they tested positive. The researchers found that two tests given within two days can indicate whether a person’s viral level is rising or falling — information that can influence treatment decisions. The findings have not yet been peer reviewed.
26 October — A maths-based strategy streamlines COVID testing
In ‘pooled’ testing for SARS-CoV-2, samples from multiple people are combined into one batch that is then analysed for the virus. Now, a large-scale trial has shown that pooled testing can be highly efficient — even more so than theory predicted.
Moran Yassour at the Hebrew University of Jerusalem and her colleagues tested 133,816 nose and throat samples by pooling either five or eight individual samples into one group sample (N. Barak et al. Preprint at medRxiv https://doi.org/ffkx; 2020). If a group tested positive, every constituent sample was retested. Groups that tested negative were not retested.
Using this method, the researchers required only one-quarter of the tests they would have needed to check every sample individually. They needed fewer tests than expected, because people from the same household, university, care home or hospital tend to get tested together, increasing the likelihood that positive samples are in the same groups. The findings have not yet been peer reviewed.
23 October — Promising drug for COVID-19 does not save lives
A study of a drug that mutes the body’s immune response found that it did not prevent the deaths of people with moderate COVID-19, dealing a blow to a once-popular hypothesis about treatments for the disease.
In some people with severe COVID-19, the immune system launches an excessive inflammatory response, suggesting a link between grave illness and an overly vigorous immune defence against SARS-CoV-2. The link is bolstered by the association between high levels of a protein called IL-6, which stimulates the immune system, and both death and the need for ventilation in people with COVID-19.
John Stone at the Massachusetts General Hospital in Boston and his colleagues tried to dampen inflammation in people with COVID-19 by treating them with the drug tocilizumab, which interferes with IL-6 activity (J. H. Stone et al. N. Engl. J. Med. https://doi.org/ffjp; 2020). But in a randomized, controlled trial of 243 people with moderate disease, the team found no statistically significant reduction in deaths or the need for ventilation among those who received tocilizumab compared with those who didn’t. The study does not rule out the possibility that a larger trial with more statistical power could uncover a benefit.
20 October — Genomics ties university COVID cases to care-home deaths
An explosive outbreak of COVID-19 among young people in a US university town spilt into the surrounding community, leading to the deaths of two people in local care homes.
Public-health officials have long warned that SARS-CoV-2 infections in young adults could easily cascade into a community’s older population. That scenario has now been confirmed in La Crosse, Wisconsin, which has three universities.
Paraic Kenny at the Gundersen Medical Foundation in La Crosse and his colleagues analysed 111 SARS-CoV-2 genomes from people in La Crosse County, where cases spiked to 2,002 in September — the month when students began in-person classes (C. S. Richmond et al. Preprint at medRxiv https://doi.org/fdt3; 2020). The team found that the “overwhelming majority” of those cases was caused by only two viral variants that spread swiftly during the first three weeks of September. Most of the cases were in people aged 17 to 29.
Clusters of young people were infected with the same variant, suggesting that the virus spread at gatherings, such as packed student parties, which took place both indoors and outdoors. One of the variants made its way into two care homes. Eight home residents were infected, and two died. The findings have not yet been peer reviewed.
19 October — The coronavirus test results that predict an outbreak’s course
Viral levels in people infected with SARS-CoV-2 in a specific town or city could be used to assess whether the epidemic there has passed its peak.
A common test for SARS-CoV-2 allows doctors to measure an infected person’s ‘viral load’, an indicator of the amount of virus in their body. James Hay at the Harvard T.H. Chan School of Public Health in Boston, Massachusetts, and his colleagues, used modelling to show that the viral loads of a population correlate with the rate of viral spread in that population (J. A. Hay et al. Preprint at medRxiv https://doi.org/ghfm73; 2020).
Early in an epidemic, the average infected person has been recently exposed to the virus and therefore has a high viral load. Later in the epidemic, the average infected person has had the virus for longer and has a low viral load.
As a result, a snapshot of the viral-load distribution in a random sample of a population can reveal whether cases in that population are on the rise or are declining, the researchers say. They add that their method is less susceptible to biases from changing COVID-testing practices than simply counting daily cases. The findings have not yet been peer reviewed.
16 October — Are rapid coronavirus tests effective? It depends
Rapid antigen tests for the coronavirus provide results within 30 minutes. But not all of the tests on the market are equally effective at detecting the virus.
Antigen-based assays detect specific surface proteins, or antigens, on SARS-CoV-2 particles, and are easy to use and inexpensive to produce. Marion Koopmans at the Erasmus University Medical Center in Rotterdam, the Netherlands, and her colleagues used 5 commercially available rapid antigen assays on samples from 1,754 people who had already tested positive for the coronavirus with the standard polymerase chain reaction test, which is highly sensitive but slow (J. van Beek et al. Preprint at medRxiv https://doi.org/fdmg; 2020).
The 2 most sensitive tests detected the virus more than 97% of the time, whereas the least sensitive test did it in about 75% of cases.
All samples came from people with COVID-19 symptoms, who tend to have high levels of coronavirus. The researchers caution that rapid antigen tests might be less effective at picking up the presence of the virus in people with low levels of virus. The finding has not yet been peer reviewed.
15 October — The coronavirus shrugs at seasonal temperature changes
The arrival of spring and summer do not slow transmission of SARS-CoV-2, say researchers who studied the early stages of the pandemic.
Influenza viruses survive for longer outside the body in cold, dry air than in warmer, more humid environments, giving them the chance to infect more people in winter than in spring and summer. Research has given a mixed picture of whether the new coronavirus shows similar behaviour.
To see how the changing seasons affected the virus’s spread in China, Canelle Poirier and Mauricio Santillana at Harvard Medical School in Boston, Massachusetts, and their colleagues created a model incorporating data from China collected between mid-January and mid-February (C. Poirier et al. Sci. Rep. 10, 17002; 2020). These data included COVID-19 case counts, weather conditions and information about domestic travel. The model also took into account lockdowns instigated by the government.
The team found that the weather alone could not explain variability in the virus’s spread, which continued in areas of China with tropical climates as well those that are cold and dry.
14 October — There’s more than one way to build a coronavirus-fighting antibody
Researchers have worked out how a range of potent immune proteins stop the new coronavirus infecting cells.
Neutralizing antibodies recognize viral particles and keep them out of cells. They are an important component of the immune system’s attack on SARS-CoV-2, and a promising experimental treatment.
A team led by Pamela Bjorkman at the California Institute of Technology in Pasadena determined the 3D shapes of eight neutralizing antibodies attached to SARS-CoV-2’s spike protein, which helps the virus to gain a foothold in host cells (C. O. Barnes et al. Nature https://doi.org/fc8d; 2020). The structures revealed that these neutralizing antibodies can be divided into several classes, according to which part of the spike protein’s cell-attachment region they recognize.
Further experiments showed that mutations that allow viruses to evade one class of neutralizing antibody are unlikely to foil others.
13 October — Common-cold antibodies offer little defence against the coronavirus
Research on archived blood does not bear out hopes that antibodies against ‘seasonal’ coronaviruses can protect against severe COVID-19.
Paul Bieniasz and Theodora Hatziioannou at the Rockefeller University in New York City and their colleagues analysed 37 blood-serum samples collected before 2020 from people in the United Kingdom (D. Poston et al. Preprint at medRxiv https://doi.org/fc4g; 2020). All of the study participants had tested positive for one of the seasonal human coronaviruses, which can cause the common cold.
The team found that each serum sample contained antibodies that could disable at least one common-cold coronavirus, blocking the virus’s ability to infect human cells in a lab dish. But the serum could not disable a hybrid virus that had been engineered to carry SARS-CoV-2’s spike protein, a crucial player in the virus’s invasion of host cells.
The results suggest that antibodies to common-cold coronaviruses do not have a major role in determining why some people with COVID-19 fare worse than others, the authors say. The findings have not yet been peer reviewed.
8 October — Dense cities should brace for long coronavirus outbreaks
The new coronavirus tears through areas where residents generally keep to their own small, close-knit communities. But the virus takes its time spreading in crowded cities where residents of different neighbourhoods tend to intermingle, ultimately infecting more people than in the relatively isolated areas.
Moritz Kraemer at the University of Oxford, UK, and his colleagues modelled the spread of SARS-CoV-2 through communities of various sizes and population densities(B. Rader et al. Nature Med. https://doi.org/fcjk; 2020). The researchers validated their model by comparing its output with known data on individual movements and infection rates in crowded Chinese cities such as Wuhan and less densely packed provinces in Italy.
The team’s model predicts relatively short, intense spikes in COVID-19 cases in relatively uncrowded cities where residents stick to their own neighbourhoods rather than mingling freely. In crowded cities, however, people are more likely to have to cope with outbreaks that last longer than do those in the countryside.
The researchers applied their model to 310 cities worldwide, and predict that those with relatively even population distributions, such as Ulaanbaatar in Mongolia, could expect a short-term explosion in cases. But more densely settled urban centres, such as Madrid, can expect more protracted outbreaks.
6 October — Teenager spreads coronavirus on family holiday
A 13-year-old girl gave the new coronavirus to her grandparents and 9 other relatives who occupied the same holiday house for up to 3½ weeks, confirming that adolescents can seed clusters of COVID-19 cases.
According to an investigation by Noah Schwartz at the Centers for Disease Control and Prevention in Atlanta, Georgia, and his colleagues, the girl was exposed to SARS-CoV-2 in June. After a rapid test suggested that she was not infected, she joined 13 family members for an extended stay in a 5-bedroom house (N. G. Schwartz et al. Morb. Mortal. Wkly Rep. https://doi.org/10.15585/mmwr.mm6940e2; 2020). Family members neither wore masks nor maintained distance from each other.
Twelve people in the house, including the teenage girl, developed COVID-19 symptoms and either tested positive for the coronavirus or were classified as probable cases. Six other relatives visited those staying in the house but remained outdoors and kept their distance. Of those six, all four who took a coronavirus test tested negative, and none fell ill.
5 October — Massive contact-tracing effort in India reveals striking trends
The patterns of infections and deaths caused by the new coronavirus differ starkly between resource-poor settings and wealthier places, according to the largest contact-tracing study conducted so far, carried out using data from India.
Joseph Lewnard at the University of California, Berkeley, and his colleagues analysed data from almost 85,000 people with COVID-19, as well as their close contacts — who numbered nearly 600,000 — in the states of Tamil Nadu and Andhra Pradesh (R. Laxminarayan et al. Science https://doi.org/10.1126/science.abd7672; 2020).
The incidence of COVID-19 in the two states declines steadily with age for people aged 40 and older — in contrast to the United States, where incidence climbs with age from age 65. Mortality rates for those aged 75 and above were markedly lower in India than in the United States — perhaps, the researchers say, because people in India who live to old age tend to be relatively wealthy compared with those who die younger.
The study also found that people were most likely to infect others within their own age group. This is especially true of children, suggesting that socialising among kids could contribute to viral spread.
2 October — The immune trait that could allow viral reinfection
Waning antibody levels or a poorly developed immune response to SARS-CoV-2 could put people at risk of reinfection, one case suggests.
In March, a care-home resident in their sixties developed severe pneumonia and tested positive for the new coronavirus. The individual spent more than one month in hospital before testing negative. In July, the individual tested positive again, with milder symptoms of coughing and shortness of breath.
Genomic analysis by Jason Goldman at the University of Washington, Seattle, and his colleagues (J. D. Goldman et al. Preprint at medRxiv https://doi.org/fbvj; 2020) showed that these were two separate infection events. The team also found that after the second infection, the individual produced only low levels of antibodies, and that these decreased over time. The person might have had a similar response to the first infection, which could explain why the individual was not protected against the second infection, the authors say.
The team also measured the individual’s neutralizing antibodies, which protect cells against infection. The person had lower levels of these potent antibodies against the version of SARS-CoV-2 that caused the first infection than against the version that caused the second infection.
The researchers say that these measurements provide a useful benchmark for antibody levels that do not protect against reinfection. The research has not yet been peer reviewed.
1 October — A fast-spreading viral variety shows higher infectiousness
Variants of SARS-CoV-2 with a widespread mutation are more infectious in human cells and hamsters, compared with viral variants lacking the change.
In February 2020, researchers examining samples from people with COVID-19 detected a SARS-CoV-2 mutation that alters the amino acid sequence of the virus’s spike protein, which the virus uses to infect cells. The amino-acid alteration, known as D614G, became common in Europe, North America and elsewhere in spring 2020, and now nearly all viruses isolated worldwide carry the alteration.
To determine the effects of the D614G change, two independent teams engineered SARS-CoV-2 particles with the mutation. Pei-Yong Shi at the University of Texas Medical Branch in Galveston, Texas and his colleagues conducted one set of experiments (J. A. Plante et al. Preprint at bioRxiv https://doi.org/fbxz; 2020); Ralph Baric at the University of North Carolina-Chapel Hill and his colleagues conducted the other (Y. J. Hou et al. Preprint at bioRxiv https://doi.org/fbxx; 2020).
Both teams found that, compared with forms of the virus that lack the mutation, D614G variants replicated more efficiently in cells from human airway tissues. Baric’s team also found that D614G variants spread faster between hamsters, which are used to study SARS-CoV-2 transmission. Neither finding has been peer reviewed yet.
30 September — A front-runner vaccine shows promise in older people
Older people injected with one of the most prominent candidate vaccines for COVID-19 developed high levels of antibodies against the new coronavirus.
Evan Anderson at the Emory University School of Medicine in Atlanta, Georgia, and his colleagues studied the response of 40 people aged 56 and above to the vaccine developed by biotechnology firm Moderna, based in Cambridge, Massachusetts, and the US National Institute of Allergy and Infectious Diseases (E. J. Anderson et al. N. Engl. J. Med. https://doi.org/fbxj; 2020). The vaccine consists of a piece of RNA that encodes a modified version of a SARS-CoV-2 protein.
Participants developed several types of antibodies — immune molecules that fight infection — including neutralizing antibodies, which can disarm an invading microbe. After receiving a second dose of the vaccine, participants had antibody levels similar to those of control-group participants who had recovered from COVID-19. Any side effects were generally mild to moderate.
28 September — Tests reveal silent reinfections in hospital workers
Two staff members at a hospital in India who tested positive for the new coronavirus became reinfected several months later — and had no symptoms in either instance.
The hospital employees, a 25-year-old-man and a 28-year-old woman, worked in the COVID-19 ward. Both tested positive for SARS-CoV-2 in May, although neither had symptoms (V. Gupta et al. Clin. Inf. Dis. https://doi.org/d97d; 2020). After testing negative, they returned to work. Both tested positive again roughly three-and-a-half months after the first positive test. Neither had symptoms, but both had higher levels of virus than in May.
Genomic analysis by Vinod Scaria at the Institute of Genomics and Integrative Biology in New Delhi and his colleagues showed that the SARS-CoV-2 that infected the workers the second time was genetically different from the first virus that infected them — evidence that the workers were infected anew rather than harbouring leftover virus.
The results suggest that asymptomatic reinfections are often underreported, the authors say.
25 September — The immune breakdown linked to dire illness
Some severe cases of COVID-19, including those in young, healthy people, could be linked to dysfunction of immune-signalling chemicals called type-1 interferons, according to a survey of nearly 1,000 people with life-threatening SARS-CoV-2 infection.
Type-I interferons are crucial for mounting a defence against influenza and other viruses. Jean-Laurent Casanova at the Rockefeller University in New York City and his colleagues analysed DNA from people with severe COVID-19, looking for specific mutations in genes that trigger production of type-I interferons (Q. Zhang et al. Science https://doi.org/d95p; 2020). The team found that 3.5% of study participants had such mutations, which rendered them unable to manufacture the signalling chemicals.
In a second study, of severely ill people, Casanova, Paul Bastard at the University of Paris and their colleagues looked for autoantibodies — antibodies that, for unknown reasons, attack the body’s own tissues and organs (P. Bastard et al. Science https://doi.org/d95q; 2020). The researchers found that more than 10% of people with severe COVID-19 had autoantibodies that targeted type-I interferon activity, compared with 0.3% in the general population. Laboratory experiments confirmed that the auto-antibodies knocked out type-I interferon activity.
The researchers suggest that interferons could be used as therapies for the disease.
24 September — Extreme infection level might have helped to quell a city’s epidemic
As much as two-thirds of the population of Manaus, a city of two million people in Brazil’s state of Amazonas, could have been infected with the new coronavirus. That’s a proportion high enough to have contributed to controlling the spread of the virus.
Ester Sabino at the University of São Paulo, Brazil, and her colleagues searched for antibodies against SARS-CoV-2 in more than 6,000 blood samples collected by a Manaus blood bank between February and August (L. F. Buss et al. Preprint at medRxiv https://doi.org/ghcm6h; 2020). From the proportion of donors who tested positive for antibodies, the authors estimate that about 66% of the population had been infected by early August — months after the epidemic in Manaus peaked in May .
The authors say that the high proportion of donors with antibodies to the virus suggests that Manaus might have reached ‘herd immunity’, the term for a scenario in which enough people are immune to an infection to control its spread.
The team says its estimate accounts for several potential sources of bias, including false positives and false negatives in antibody testing. The findings have not yet been peer reviewed.
22 September — Good timing might help the immune system to control COVID-19
People aged 65 and older who are infected with the new coronavirus tend to mount a disorganized immune response — a response that is also associated with severe COVID-19. This could help to explain why the disease strikes older people particularly hard.
The immune system’s ‘adaptive’ branch, which targets specific invaders, has three principle components: antibodies, CD4+ T cells and CD8+ T cells. Alessandro Sette and Shane Crotty at the La Jolla Institute for Immunology in California studied the adaptive immune response in 24 people whose COVID-19 symptoms ranged from mild to fatal (C. R. Moderbacher et al. Cell https://doi.org/ghbwh7; 2020).
The team found that people whose immune systems failed to rapidly launch the entire adaptive immune system tended to have more severe disease than did people in whom all three arms ramped up production simultaneously. An uncoordinated response was particularly common among older people, and could indicate that both antibodies and T cells are important weapons against the coronavirus.
21 September — Business-class passenger spreads coronavirus on flight
Genetic evidence strongly suggests that at least one member of a married couple flying from the United States to Hong Kong infected two flight attendants during the trip.
Researchers led by Leo Poon at the University of Hong Kong and Deborah Watson-Jones at the London School of Hygiene & Tropical Medicine studied four people on the early-March flight (E. M. Choi et al. Emerg. Infect. Dis. https://doi.org/d9jn; 2020). Two were a husband and wife travelling in business class. The others were crew members: one in business class and one whose cabin assignment is unknown. The passengers had travelled in Canada and the United States before the flight and tested positive for the new coronavirus soon after arriving in Hong Kong. The flight attendants tested positive shortly thereafter.
The team found that the viral genomes of all four were identical and that their virus was a close genetic relative of some North American SARS-CoV-2 samples — but not of the SARS-CoV-2 prevalent in Hong Kong. This suggests that one or both of the passengers transmitted the virus to the crew members during the flight, the authors say. The authors add that no previous reports of in-flight spread have been supported by genetic evidence.
18 September — Musicians and a monk are tied to superspreading in Hong Kong
An estimated 19% of SARS-CoV-2 infections in Hong Kong seeded 80% of the local transmission of the virus from one person to another, according to an analysis of the virus’s early spread. The analysis also found that viral spread in social settings caused more infections than spread within family households.
In an examination of more than 1,000 coronavirus infections in Hong Kong from late January to late April, Peng Wu at the University of Hong Kong and her colleagues found evidence of multiple ‘superspreading’ events, in which one infected person passed the virus to at least six others (D. C. Adam et al. Nature Med. https://doi.org/d9c4; 2020). Musicians who performed at four Hong Kong bars are thought to have triggered the biggest cluster, which led to 106 cases. Another 19 cases were linked to a temple; one monk there had no symptoms but was found to be infected.
Nearly 70% of the cases did not transmit to anyone, the team found. The analysis also showed that more downstream cases were linked to spread in social settings such as weddings and restaurants than to household spread.
17 September — Immunity to common-cold coronaviruses is short-lived
Natural immunity to coronaviruses that cause the common cold might last for only a few months after infection, according to a study that monitored volunteers’ antibody levels — some for more than three decades.
Previous studies have suggested that immune responses to common-cold coronaviruses protect against reinfection for only a matter of months, although symptoms are often reduced during the second infection. Lia van der Hoek at the University of Amsterdam and her colleagues looked for coronavirus antibodies in blood samples taken every few months from ten individuals, starting in the mid-1980s (A. W. D. Edridge et al. Nature Med. https://doi.org/ghbm79; 2020).
The team used a rise in antibody levels as an indicator of infection. Infections with coronaviruses were least common from June to September, a seasonal pattern that the authors suggest SARS-CoV-2 might follow. The authors found reinfections occurring as early as 6 months after the first infection, and most often at 12 months.
15 September — A groundbreaking guide to making ‘cocktails’ to treat COVID-19
A new method pinpoints every mutation that a crucial SARS-CoV-2 protein could use to evade an attacking antibody. The results could inform the development of antibody treatments for COVID-19.
The immune system produces molecules called antibodies to fend off invaders. Antibodies that bind to an important region of the SARS-CoV-2 spike protein can inactivate the viral particles, making such antibodies attractive as therapies. But over time, viruses can accumulate mutations — and some can interfere with antibody binding and allow viral particles to ‘escape’ immune forces.
James Crowe at the Vanderbilt University Medical Center in Nashville, Tennessee, Jesse Bloom at the Fred Hutchinson Cancer Center in Seattle, Washington, and their colleagues created the most detailed map so far of the spike-protein mutations that could prevent binding by ten human antibodies (A. J. Greaney et al. Preprint at bioRxiv https://doi.org/d8zm; 2020). The team then used that information to design three antibody cocktails, each consisting of two antibodies.
In laboratory tests of the cocktails against SARS-CoV-2, the virus did not develop mutations that could escape antibody binding. The findings have not yet been peer reviewed.
14 September — Kids in US childcare centres spread coronavirus to families
Twelve children infected with the new coronavirus at childcare centres passed the virus on to at least another twelve people between them, according to an analysis of outbreaks in Utah. Among the resulting cases was a woman who had to be hospitalized after presumptive infection by her child.
Cuc Tran at the US Centers for Disease Control and Prevention in Atlanta, Georgia, and her colleagues investigated outbreaks at three childcare centres in Salt Lake County (Morb. Mortal. Wkly Rept. https://www.cdc.gov/mmwr/volumes/69/wr/mm6937e3.htm?s_cid=mm6937e3_w; 2020). At all three centres, the first known case was a staff member. Two had gone to work even though a person in their household had shown COVID-19 symptoms.
All 12 infected children, whose ages ranged from 8 months to 10 years, had either mild or no symptoms. Among the children’s close contacts who tested positive were six mothers and three siblings; one eight-month-old baby infected both parents. Not all close contacts were tested, meaning that infections associated with the childcare centres might have been missed, the authors say.
11 September — Nearly half of coronavirus transmission is from people not yet feeling ill
Some three-quarters of incidents of SARS-CoV-2 transmission occur in the few days before or after the onset of symptoms in the person who passes on the virus.
Luca Ferretti at the University of Oxford, UK, and colleagues studied 191 cases of SARS-CoV-2 transmission from an infected person to an uninfected person. The team analysed the timing of the transmitting person’s initial infection and onset of symptoms, and when that person spread the infection to someone else (L. Ferretti et al. Preprint at medRxiv https://doi.org/d8ms; 2020).
They found that roughly 40% of transmission events occurred before the onset of symptoms, and around 35% took place on the day that symptoms appeared or on the following day.
The researchers say their findings underscore the importance of mass testing, contact tracing and physical distancing to prevent transmission from pre-symptomatic people, as well as self-isolation for at least two days at the first sign of symptoms such as cough, fever, fatigue and loss of smell — however mild.
10 September — Surprise! A host of tantalizing new SARS-CoV-2 proteins is unveiled
Researchers have discovered nearly two dozen previously unknown proteins encoded by SARS-CoV-2 — and their role during infection is mostly mysterious.
Until now, SARS-CoV-2’s RNA genome was known to hold the instructions for making 29 proteins, such as the spike protein that helps viral particles to infect cells, and a variety of viral proteins that become active inside cells. But scientists were uncertain whether the virus had more than those 29.
To identify further proteins, Noam Stern-Ginossar at the Weizmann Institute of Science in Rehovot, Israel, and her colleagues sequenced SARS-CoV-2 RNA bound to protein-making machines called ribosomes inside infected cells (Y. Finkel et al. Nature https://doi.org/d8pb; 2020). This scan turned up 23 previously unknown proteins, including some that are entirely new and others that are shortened or extended versions of known proteins.
Some of the newfound proteins might control production of known viral molecules, but the role of many is unknown.
9 September — The immune-cell traits that could predict severe COVID-19
Immune cells called neutrophils are more likely to be primed for action in people who will eventually develop severe COVID-19 than in those who are will go on to become only mildly ill, according to a machine-learning analysis of data from 3,300 people. If the results can be reproduced, they could aid early identification of the people most likely to become critically ill.
Neutrophils comprise an important part of the body’s rapid response to infection, but can also damage uninfected tissue. Hyung Chun of Yale University in New Haven, Connecticut, and his colleagues used machine learning to analyse proteins in blood plasma taken from people hospitalized with COVID-19 (M. L. Meizlish et al. Preprint at medRxiv https://doi.org/d8hm; 2020).
Several immune proteins that are associated with neutrophils were found at higher levels in the plasma of people who later became critically ill than in those whose illness did not become severe. A subsequent analysis of health records from about 3,300 people showed that high neutrophil counts were associated with increased COVID-19 mortality. The findings have not yet been peer reviewed.
8 September — Kids ravaged by COVID-19 show unique immune profile
Most children infected with the new coronavirus show few signs of illness, if any. But a few children are struck by a severe form of COVID-19 that can cause multiple organ failure and even death. Now, scientists have begun to tease out the biology of this rare and devastating condition, called multisystem inflammatory syndrome in children, or MIS-C.
Doctors have diagnosed hundreds of cases of MIS-C, which shares some similarities with the childhood illness Kawasaki’s disease. To understand MIS-C’s biological profile, Petter Brodin at the Karolinska Institute in Stockholm and his colleagues looked at 13 children with MIS-C, 28 children with Kawasaki’s disease and 41with mild COVID-19 (C. R. Consiglio et al. Cell https://doi.org/d8fh; 2020). The researchers found that compared with children with Kawasaki’s disease, those with MIS-C have lower levels of an immune chemical called IL-17A, which has been implicated in inflammation and autoimmune disorders.
Unlike all the other children studied, children with MIS-C had no antibodies to two coronaviruses that cause the common cold. This deficit might be implicated in the origins of their condition, the authors say.
4 September — Powerful new evidence links steroid treatment to lower deaths
People severely ill with COVID-19 are less likely to die if they are given drugs called corticosteroids than people who are not, according to an analysis of hospital patients on five continents.
Earlier findings showed that the steroid dexamethasone cut deaths in people with COVID-19 on ventilators. To examine the effects of steroids in general, Jonathan Sterne at the University of Bristol, UK, and his colleagues did a meta-analysis that pooled data from seven clinical trials; each of the seven studied the use of steroids in people who were critically ill with COVID-19 (REACT Working Group J. Am. Med. Assoc. https://doi.org/d7z8; 2020). The trials included more than 1,700 people across 12 countries.
The team analysed participants’ status 28 days after they were randomly assigned to take either a steroid or a placebo. The risk of death was 32% for those who took a steroid and 40% for those who took a placebo. The authors say that steroids should be part of the standard treatment for people with severe COVID-19.
3 September — In a first, genomics shows that mink can pass SARS-CoV-2 to humans
An investigation of Dutch mink farms has found the first documented cases of animal-to-human transmission of SARS-CoV-2.
After SARS-CoV-2 outbreaks among farmed mink were first detected in late April, Marion Koopmans at Erasmus Medical Centre in Rotterdam, the Netherlands, and her colleagues used genome sequencing to track outbreaks among animals and workers at 16 mink farms (B. B. O. Munnink et al. Preprint at bioRxiv https://doi.org/d7xn; 2020). The team tested 97 farmworkers and their contacts, and found evidence for SARS-CoV-2 infection in 66 of them.
Genetic analysis suggested that workers had introduced SARS-CoV-2 to mink, which spread the virus back to workers, who might then have passed it on to other people. Outbreaks at mink farms have been detected in Denmark, Spain and the United States, and the researchers say unchecked spread could lead to the animals becoming a reservoir for human infections. The findings have not yet been peer reviewed.
2 September — Antibodies persist for months rather than dwindling
A sweeping survey in Iceland shows that antibodies against the new coronavirus endure in the body for four months after infection, countering earlier evidence suggesting that these important immune molecules quickly disappear.
After a pathogen invades, the immune system produces proteins called antibodies to fight off the intruder. Scientists do not know whether people who generate antibodies against SARS-CoV-2 are protected from reinfection, nor do they know how long those antibodies persist.
Kari Stefansson at deCODE Genetics–Amgen in Reykjavik and his colleagues measured the levels of SARS-CoV-2 antibodies in the blood of roughly 30,000 people, including more than 1,200 who had tested positive for the virus and recovered from COVID-19 (D. F. Gudbjartsson et al. N. Engl. J. Med. https://doi.org/gg9hbt; 2020). Roughly 90% of the recovered people had antibodies against the virus. Their antibody levels rose during the two months after diagnosis, plateaued and then remained at the same level for the duration of the study.
The results also show that the virus has infected only 0.9% of the population, leaving Iceland “vulnerable to a second wave of infection”, the authors warn.
1 September — Even octogenarians develop potent antibodies
As the new coronavirus ripped through several care homes in England, more than 80% of the residents mounted an antibody response to the virus, including 82% of those over the age of 80.
During outbreaks at six residential and nursing homes, Shamez Ladhani at Public Health England in London and his colleagues tested more than 500 residents and staff for SARS-CoV-2 infection (S. N. Ladhani et al. Preprint at medRxiv https://doi.org/d7p2; 2020). About five weeks later, the team tested many of the same people for antibodies to SARS-CoV-2 and in particular for neutralizing antibodies, potent molecules that can block the virus from infecting cells
The team found that roughly the same proportion of staff members and care-home residents had formed antibodies to the coronavirus. And neutralizing antibodies had developed in almost 90% of both staff members and residents, including more than 80% of people over the age of 80.
The authors caution that it is not clear whether antibodies against the virus guard against reinfection. The findings have not yet been peer-reviewed.
28 August ― COVID-19 testing helps sleep-away summer camps to avoid outbreaks
Rigorous SARS-CoV-2 testing and infection-control measures prevented outbreaks at four overnight camps in Maine that hosted hundreds of children between mid-June and mid-August.
Laura Blaisdell at the Maine Medical Center in Portland and colleagues report that the four sleep-away camps asked all attendees — both campers and staff — to be tested for SARS-CoV-2 before arrival (L. L. Blaisdell et al. Morb. Mortal. Wkly Rep. https://www.cdc.gov/mmwr/volumes/69/wr/mm6935e1.htm?s_cid=mm6935e1_w; 2020). Shortly after arrival, attendees were re-tested for the virus. They were also assigned to small cohorts and spent the first 14 days of camp quarantining with members of their cohort.
Of more than 1,000 attendees, 2 staff members and one camper tested positive at camp and were isolated until they tested negative. The 30 people in the camper’s cohort were quarantined; all tested negative for the virus during quarantine. The authors say that the virus did not spread beyond the three infected attendees.
27 August — Why infected primary-school pupils could be hard to spot
Children aged 6 to 13 are less likely to have symptoms of COVID-19 than those who are younger or older, according to a study of nearly 400 infected people under the age of 21.
Matthew Kelly and his colleagues at Duke University School of Medicine in Durham, North Carolina, studied 382 children and young adults who had had close contact with a person infected with SARS-CoV-2 (J. H. Hurst et al. Preprint at medRxiv http://doi.org/d7cb; 2020). Roughly three-quarters of the study participants tested positive for SARS-CoV-2 either before or during the study.
Only 61% of infected children aged 6 to 13 showed symptoms, compared with 75% of infected study participants under age 6 and 76% of those over age 13. Children aged 6–13 who did feel ill tended to have milder symptoms than older and younger study participants.
Nearly one-third of infected children with an infected sibling did not have close contact with an infected adult, implying that the virus had spread from child to child.
Screening systems at schools and day-care centres should account for age-related differences in symptoms, the authors say. The results have not yet been peer reviewed.
26 August — Sex differences in the COVID-19 immune response might drive men’s high risk
Variations in the immune response to SARS-CoV-2 could explain why men are more likely to be hospitalized and die of COVID-19 than are women.
Akiko Iwasaki at Yale University School of Medicine in New Haven, Connecticut, and colleagues studied the immune responses of 98 men and women infected with SARS-CoV-2. All had mild to moderate symptoms (T. Takahashi et al. Nature http://doi.org/d7gb; 2020). The researchers noticed that male participants’ typical immune response to infection differed from that of female participants, which could explain the more severe disease often observed in men. (Nature recognizes that sex and gender are neither binary nor fixed.)
The team found that in general, men had higher levels of certain inflammation-causing proteins known as cytokines and chemokines circulating in their blood than had women. By contrast, women tended to have a stronger response from immune cells known as T cells than did men. In men, an increase in symptom severity over time was associated with a weak T-cell response; in women, it was associated with increased amounts of inflammatory cytokines.
The study proposes taking sex into account when treating people with COVID-19.
25 August ― Reinfection with SARS-CoV-2 is confirmed for the first time with genetic evidence
A man in Hong Kong who was ill with COVID-19 in March was infected by a different variant of the new coronavirus several months later — the first evidence for reinfection that is supported by genetic analysis.
People infected with SARS-CoV-2 mount an immune response, which scientists think probably prevents most reinfections. The durability of this protection is unclear, and a documented case of reinfection would signal that immunity can wane. But previously reported reinfections have been found to relate instead to prolonged shedding of the virus or its genetic material
Kwok-Yung Yuen and his colleagues at the University of Hong Kong identified a 33-year-old man who recovered from COVID-19 in April and tested positive again more than 4 months later, after returning from Spain via the United Kingdom (K. K.-W. To et al. Clin. Infect. Dis. http://doi.org/d7ds; 2020). Genetic sequencing suggested that the second infection was caused by a virus that was genetically distinct from the one responsible for his initial bout.
The man never developed symptoms from the second infection, but his immune system responded by producing a fresh batch of antibodies.
21 August — Vaccines given through the nose could protect against infection
Studies in mice and monkeys show that nasal vaccinations can shield the animals from the new coronavirus — and that such vaccinations might be more effective than an injected form of the same vaccine.
David Curiel and Michael Diamond at Washington University School of Medicine in St Louis, Missouri, and their colleagues created a candidate vaccine encoding the SARS-CoV-2 spike protein, which the virus uses to invade cells (A. O. Hassan et al. Cell http://doi.org/d63k; 2020). The researchers then gave the vaccine to bioengineered mice that had human receptors for the protein.
After being injected with the vaccine and then exposed to SARS-CoV-2, mice showed no infectious virus in their lungs — but their lungs did harbour small amounts of viral RNA. By contrast, mice that had the vaccine inserted up their noses before exposure had no measurable viral RNA in their lungs. This and other evidence suggests that the nasal vaccine entirely warded off infection, the authors say.
Ling Chen at the First Affiliated Hospital of Guangzhou Medical University in China and colleagues developed another vaccine encoding the spike protein (L. Feng et al. Nature Commun. 11, 4207; 2020). The researchers found that both nasal and injected forms of the vaccine protected rhesus macaques (Macaca mulatta) from infection. The authors say that a vaccine that can be given by nose might allow people to vaccinate themselves.
20 August — A coronavirus mutation is tied to less severe illness
A SARS-CoV-2 mutation that appeared in East Asia early in the pandemic is linked to symptoms milder than those caused by the unmutated version of the virus.
In early 2020, researchers in Singapore identified a cluster of COVID-19 cases caused by a SARS-CoV-2 variant missing a chunk of RNA that spanned two genes, ORF7b and ORF8. To determine the consequences of this change, called a deletion, Lisa Ng at the Singapore Immunology Network and colleagues compared people infected with viruses carrying the deletion with those infected by normal viruses (B. E. Young et al. Lancet http://doi.org/d6x7; 2020).
None of the 29 people whose viruses had the mutation needed supplemental oxygen, but 26 of the 92 people whose viruses lacked the mutation did. Viruses carrying the deletion haven’t been detected since March — possibly owing to infection-control measures.
The virus responsible for the 2002–04 outbreak of severe acute respiratory syndrome (SARS) acquired a similar deletion in the ORF8 gene, suggesting that this might be an important adaption to infecting humans, the authors say.
Correction: An earlier version of this article said researchers identified a SARS-CoV-2 variant missing a chunk of DNA.
19 August — An unprecedented map charts a key viral protein
For the first time, researchers have mapped the 3D shape of spike proteins that are part of intact SARS-CoV-2 particles.
Spike proteins decorate the surface of coronaviruses and lock onto host receptors, such as ACE2, to gain entry to cells. The first structures of SARS-CoV-2’s spike were gleaned from modified proteins that had been expressed in cells and then purified. To check these models John Briggs at the Medical Research Council Laboratory of Molecular Biology in Cambridge, UK, and colleagues collected viral particles from infected cells and determined the shape of their spike proteins using electron microscopy (Z. Ke et al. Nature http://doi.org/d6sf; 2020).
These structures closely resembled the ones determined from purified forms. In both, the spike protein can adopt either a ‘closed’ confirmation or an ‘open’ one, which allows it to bind to a receptor. Studying the structure in viral particles could help to explain how spike-binding antibodies block infection, the researchers say.
17 August — Sailors furnish first evidence that antibodies protect humans against re-infection
A massive COVID-19 outbreak on a US fishing boat spared crew members who already had antibodies against the new coronavirus, providing what scientists say is the first direct evidence that these antibodies protect people against being reinfected.
After a viral infection, the immune system makes compounds called neutralizing antibodies that can attack the virus if it invades again. But previous research had not determined whether such antibodies can shield humans from SARS-CoV-2 reinfection.
Alexander Greninger at the University of Washington School of Medicine in Seattle and his colleagues tested the crew of a US fishing vessel for SARS-CoV-2 and for antibodies to the virus (A. Addetia et al. Preprint at medRxiv http://doi.org/d6qm; 2020). Just before the ship’s departure, the researchers tested 120 of the 122 crew members and found that all were negative for SARS-CoV2, but an outbreak hit the ship soon after it left shore.
Post-voyage testing showed that 104 members of the 122-person crew were infected. None of those who were infected and had been tested before embarking had shown neutralizing antibodies against SARS-CoV-2.
But all three crew members who did have such antibodies before departure escaped infection, providing statistically significant evidence that neutralizing antibodies acquired during SARS-CoV-2 infection protect against reinfection, the authors say. The findings have not yet been peer reviewed.
7 August — For fast and low-cost COVID-19 testing, just spit
A quick, cheap and painless test that detects SARS-CoV-2 RNA in spit could be used for mass testing.
Chantal Vogels at Yale School of Medicine in New Haven, Connecticut, and colleagues developed a simple saliva test — called SalivaDirect — to address the growing demand for extensive testing as lockdowns lift (C. B. F. Vogels et al. Preprint at medRxiv http://doi.org/d5s3; 2020).
Compared with the gold-standard nose and throat swab, the saliva test is less invasive, does not need to be conducted by a trained professional and avoids the use of scarce chemicals that are needed to store and extract viral RNA. In validation experiments, SalivaDirect detected 32 out of 34 samples that tested positive in nose and throat swabs, and 30 out of 33 negative samples.
The researchers estimate a cost-per-spit of US$1.29–$4.37, and have requested that the United States Food and Drug Administration authorize the test for emergency use.
6 August — Immune reaction to some common colds might provide protection
Some immune cells that recognize coronaviruses that cause the common cold also respond to SARS-CoV-2, the coronavirus responsible for the COVID-19 pandemic.
Previous studies have found that some people who have never been exposed to SARS-CoV-2 nevertheless have immune cells called memory T cells that can recognize the virus. Daniela Weiskopf and Alessandro Sette at the La Jolla Institute for Immunology in California analysed such T cells, and found that they recognize particular sequences of several SARS-CoV-2 proteins (J. Mateus et al. Science http://doi.org/d5v5; 2020).
The team then identified similar sequences in common-cold coronaviruses, and showed these sequences could activate some T cells that also respond to SARS-CoV-2. The findings add weight to the hypothesis that existing immunity to cold coronaviruses could contribute to differences in COVID-19 severity, but further studies are required to support that conclusion.
5 August — Antibody blend protects monkeys and hamsters from viral symptoms
A mixture of two human antibodies against the new coronavirus shows promise in animal tests for preventing and treating COVID-19.
Neutralizing antibodies are immune molecules that can attach to viruses and disable them. Christos Kyratsous at Regeneron Pharmaceuticals in Tarrytown, New York, and his colleagues made a cocktail of two neutralizing antibodies that bind SARS-CoV-2. They gave the cocktail to rhesus macaques (Macaca mulatta), which become mildly ill when infected.
The researchers found that compared to animals that took a placebo, monkeys that received the antibody combination were less likely to develop pneumonia and, if they did, had less lung damage. This was true in monkeys that took the antibodies either before or after receiving a dose of the virus (A. Baum et al. Preprint at bioRxiv http://doi.org/d5r9; 2020).
Unlike macaques, Syrian golden hamsters (Mesocricetus auratus) infected with SARS-CoV-2 become acutely ill. But hamsters dosed with virus lost less weight — or even gained weight — compared with control rodents if given the antibody cocktail before or after receiving a dose of the virus. The findings have not yet been peer reviewed.
3 August — Summer-camp outbreak infects more than 200 children
Despite measures to prevent the spread of the new coronavirus, at least 250 campers and staff members tested positive for SARS-CoV-2 after attending an overnight camp in the US state of Georgia.
Christine Szablewski at the Georgia Department of Public Health in Atlanta and her colleagues investigated the outbreak, which began two days after the first campers’ arrival on 21 June (C. M. Szablewski et al. Morb. Mortal. Wkly Rep. http://doi.org/d5ms; 2020). All campers and staff were required to test negative for the virus fewer than 13 days before arrival, and campers did not mix with those sleeping in other cabins. Campers were not required to wear masks.
The researchers found that nearly 100 staff members — many of them teenagers — tested positive in the two weeks after leaving camp. So did 168 campers, including half of those aged between 6 and 10. Factors contributing to the outbreak included the large number of campers sleeping in each cabin and what the researchers describe as “daily vigorous singing and cheering”.
30 July — Vaccine candidate protects monkeys from infection
An experimental coronavirus vaccine seems to have completely prevented infection in most monkeys that received the jab.
Hanneke Schuitemaker at Janssen Vaccines and Prevention in Leiden, the Netherlands, Dan Barouch at Beth Israel Deaconess Medical Center in Boston, Massachusetts, and their colleagues gave 32 rhesus macaques (Macaca mulatta) a single dose of one of 7 vaccines (N. B. Mercado et al. Nature http://doi.org/d5d4; 2020). Each vaccine comprised a weakened respiratory virus coding for one of seven forms of SARS-CoV-2’s spike protein.
After vaccination, nearly all the monkeys made neutralizing antibodies — powerful immune molecules that can block infection — and T cells that trigger other immune responses. When monkeys were exposed to SARS-CoV-2, the most potent of the vaccines prevented lung infection in six out of six animals that received it, and nasal infection in five out of six.
Across all the vaccinated monkeys, levels of neutralizing antibodies were associated with protection from SARS-CoV-2 infection, but levels of T cells were not.
29 July — Immune cells against the virus are found in unexposed people
Immune cells called T cells are prepared to attack the new coronavirus not only in people with COVID-19, but also in some who have not been exposed to the virus.
At first, researchers studying the immune response to SARS-CoV-2 focused mostly on the immune molecules called antibodies, but T cells offer another possible route to immunity. Andreas Thiel at Charité University Hospital Berlin and his colleagues surveyed blood samples for T cells that react to the SARS-CoV-2 spike protein (J. Braun et al. Nature http://doi.org/d5bv; 2020).
The team found such cells in 83% of study participants with COVID-19, as well as 35% of healthy blood donors who had not been exposed to SARS-CoV-2. The authors speculate that the reactive T cells might have been generated in healthy donors during past infections with related coronaviruses, but it remains unclear whether these cells offer protection against SARS-CoV-2.
28 July — Mutations allow virus to elude antibodies
Mutations in SARS-CoV-2 might help the virus to thwart potent immune molecules.
The blood of many people who recover from COVID-19 contains immune-system molecules called neutralizing antibodies that disable particles of the new coronavirus. Most such antibodies recognize the new coronavirus’s spike protein, which the virus uses to infect cells. Researchers hope that these molecules can be used as therapies, and can be elicited by vaccines.
Theodora Hatziioannou and Paul Bieniasz at the Rockefeller University in New York City and their colleagues engineered a version of the vesicular stomatitis virus, which infects livestock, to make the spike protein. They then grew the virus in the presence of neutralizing antibodies (Y. Weisblum et al. Preprint at bioRxiv http://doi.org/d439; 2020). The spike protein in the engineered viruses acquired mutations that allowed the viruses to escape recognition by a range of neutralizing antibodies.
The team also found these mutations in SARS-CoV-2 samples from infected people around the world, although at very low frequencies. Treatment ‘cocktails’ of multiple neutralizing antibodies, each recognizing a different part of the spike protein, could stop the virus from evolving resistance to these molecules, the authors suggest. The findings have not yet been peer reviewed.
27 July — The power of China’s virus-control campaign is seen in pattern of symptoms
In China, a key metric of epidemics called the serial interval shrank drastically soon after the new coronavirus’s arrival — a finding that underscores the success of China’s testing and isolation efforts.
The serial interval is the average time between the onset of symptoms in a chain of people infected by a pathogen. Benjamin Cowling at the University of Hong Kong and his colleagues modelled the spread of SARS-CoV-2 in China and found that the serial interval plummeted from 7.8 days to 2.6 days over a 5-week period starting on 9 January (S. T. Ali et al. Science http://doi.org/gg5mpc; 2020).
The researchers say that early isolation of cases prevented transmission that would otherwise have occurred later in an infectious period, leading to fewer cases and slowing the spread of the virus. As a result, most of the remaining transmissions occurred either before infected people showed symptoms or early in the symptomatic phase, and the serial interval shrank.
The authors suggest the serial interval distribution be used in real time to track the changing transmissibility of the virus.
24 July — Dogs’ and cats’ infection rates mirror those of people
Cats and dogs are just as likely to be infected with SARS-CoV-2 as people are, according to a survey in northern Italy that is the largest study of pets so far.
Nicola Decaro at the University of Bari and his colleagues took nose, throat or rectal swabs of 540 dogs and 277 cats in northern Italy between March and May (E. I. Patterson et al. Preprint at bioRxiv http://doi.org/d4r7; 2020). The animals lived in homes with infected people, or in regions severely affected by COVID-19.
None of the pets tested positive for SARS-CoV-2 viral RNA, but in further tests of antibodies against the virus circulating in the blood of some animals, the researchers found that around 3% of dogs and 4% of cats showed evidence of previous infection.
Infection rates among cats and dogs were comparable with those among people in Europe at the time of testing, suggesting that it is not unusual for pets to be infected. The findings have not yet been peer reviewed.
24 July — Virus rips through Israeli school after masking is suspended
More than 150 students at an Israeli secondary school were infected by the new coronavirus after students were allowed to remove their masks during a heat-wave.
Roughly 10 days after Israeli schools fully reopened on 17 May, two students at a secondary school in Jerusalem were diagnosed with COVID-19. Chen Stein-Zamir at the Ministry of Health in Jerusalem and her colleagues investigated the resulting outbreak and found that 153 students and 25 members of staff had become infected (C. Stein-Zamir et al. Euro Surveill. http://doi.org/d4sw; 2020). By mid-June, a further 87 cases had occurred among the close contacts of people infected through the school outbreak.
The virus’s spread was probably aided by a heat-wave that occurred between 19 and 21 May, prompting heavy use of air-conditioning and a suspension of the requirement that students wear face masks. Crowding might also have contributed: each of the school’s classrooms held 35 to 38 students, resulting in space allotments of 1.1–1.3 square metres per student.
22 July — Severely ill people yield diverse trove of powerful antibodies
Scientists have identified a diverse group of antibodies that block the new coronavirus’s ability to infect cells — even when applied in low doses.
The immune-system proteins called neutralizing antibodies interfere with hostile microbes trying to enter target cells. David Ho at Columbia University Vagelos College of Physicians and Surgeons in New York City and his colleagues studied neutralizing antibodies from the plasma of five people with severe cases of COVID-19 (L. Liu et al. Nature http://doi.org/d4md; 2020).
Nineteen antibodies proved highly effective at preventing SARS-CoV-2 infection of cell samples. A small dose of one of the antibodies protected golden Syrian hamsters (Mesocricetus auratus) from SARS-CoV-2 infection.
The 19 antibodies attach to a variety of locations on the coronavirus spike protein. A therapy made from antibodies that fasten onto the spike protein at multiple sites could be difficult for the virus to evade through mutation.
21 July — Viral levels could help to target treatment
The amount of viral RNA in the nose and throat of a person infected with the new coronavirus could help clinicians to decide how best to treat them, according to an analysis of thousands of swabs taken at a hospital in Switzerland.
Onya Opota and his colleagues at Lausanne University Hospital analysed the viral load — the amount of virus in a standard volume of material — of samples taken from 4,172 people infected with SARS-CoV-2 between 1 February and 27 April (D. Jacot et al. Preprint at medRxiv http://doi.org/d4b8; 2020). They noticed two distinct stages of COVID-19. Early in the disease, people have high viral loads, which tend to decline gradually as the disease progresses. This later stage is typically characterized by inflammation. The decline of viral loads could thus serve as a cue to start treating infected people with anti-inflammatory drugs.
But the researchers found no correlation between viral load and the severity of disease, suggesting that it is not a good predictor of a patient’s outcome. The research has not yet been peer reviewed.
16 July — Antiviral antibodies peter out within weeks after infection
Key antibodies that neutralize the effects of the new coronavirus fall to low levels within months of SARS-CoV-2 infection, according to the most comprehensive study yet.
Neutralizing antibodies can block a pathogen from infecting cells. But such antibody responses against coronaviruses often wane after just a few weeks.
Katie Doores at King’s College London and her colleagues monitored the concentration of neutralizing antibodies against SARS-CoV-2 in 65 infected people for up to 94 days (J. Seow et al. Preprint at medRxiv http://doi.org/d3s2; 2020). In a preprint that has not yet been peer reviewed, the team reports that at the peak of antibody production, people with severe COVID-19 symptoms had higher levels of antibodies than had people with mild disease.
However, in most people, antibody levels began to fall about a month after symptoms appeared, sometimes to nearly undetectable levels — raising questions about the durability of vaccines designed to promote the production of neutralizing antibodies.
15 July — Positive trial results raise hopes for a top vaccine candidate
A leading COVID-19 vaccine candidate generates an immune response against the virus and causes few side effects, according to preliminary data from a phase I safety study with 45 participants.
The vaccine is being co-developed by Moderna in Cambridge, Massachusetts, and the US National Institute of Allergy and Infectious Diseases. It consists of RNA instructions that prompt human cells to make the virus’s spike protein, generating an immune response.
Lisa Jackson at Kaiser Permanente Washington Health Research Institute in Seattle and her colleagues gave participants two injections, administered four weeks apart, of one of three different doses of the vaccine (L. A. Jackson et al. N. Engl. J. Med. http://doi.org/d3tt; 2020). Most side effects were mild, although three participants who got the highest dose experienced worse complications, such as a high fever.
After the injections, all participants produced immune proteins called antibodies capable of recognizing the SARS-CoV-2 virus, as well as ‘neutralizing antibodies’ that can block infection. A 30,000-participant phase III trial to test whether the vaccine can prevent COVID-19 is set to begin in late July.
15 July — Severe COVID-19 has a telltale immune profile
Scientists have identified an immune-system signature in people with serious COVID-19 — a finding that could inform the development of treatments for the disease.
Benjamin Terrier at the University of Paris and his colleagues analysed blood samples from 50 people infected with SARS-CoV-2 (J. Hadjadj et al. Science http://doi.org/gg4vjx; 2020). Compared to the individuals with mild or moderate symptoms, those with severe disease produced fewer antiviral proteins called interferons and more inflammatory molecules. The researchers also found that blood levels of a specific interferon decreased just before participants had to be taken to intensive-care units.
The results suggest that reduction of interferon levels in the blood is a hallmark of severe COVID-19. Treatments that counter inflammation and increase levels of interferons could help people with the disease, the researchers say.
13 July — Virus’s US invasion might have started in 2019
The new coronavirus spread across much of the interior of the United States by tagging along with people moving from state to state, but US coastal regions were seeded with SARS-CoV-2 imported from other countries — perhaps in 2019, according to models.
Alessandro Vespignani at Northeastern University in Boston, Massachusetts, and his colleagues studied air traffic, commuting patterns and other data to understand how and when the coronavirus took hold in the United States (J. T. Davis et al. Preprint at medRxiv http://doi.org/d3mf; 2020). The team found that in several coastal states, international travel drove introduction of the virus. In California and New York, SARS-CoV-2 might have begun circulating as early as December 2019.
But in many non-coastal states, domestic travellers rather than international visitors were the source of the first wave of infections. Infections spread across the country from late January to early March but were largely undetected, the authors say. The findings have not yet been peer reviewed.
10 July — Massive contact-tracing effort finds hundreds of cases linked to nightclubs
Mobile phone and credit card data helped to identify nearly 250 coronavirus infections linked to a fast-moving outbreak that began in a popular nightclub district in Seoul.
Soon after South Korean nightclubs reopened 30 April, public-health officials noted a cluster of COVID-19 cases among people who had visited Seoul’s Itaewon club district. Jin Yong Lee at Seoul National University Boramae Medical Centre and his colleagues used mobile phone location data, credit card payment records and other information to identify more than 60,000 people who had spent time in or near Itaewon clubs (C. R. Kang et al. Emerg. Infect. Dis. http://doi.org/gg4fhj; 2020) in late April or early May. All were encouraged to undergo testing for SARS-CoV-2.
By late May, officials had tested more than 40,000 people. The effort turned up 246 infections — including several that were 3, 4 and even 5 steps along the transmission chain from club-goers.
9 July — University infections could soar even if students were tested weekly
To safely reopen residential campuses, universities might need to test their students for COVID-19 every two days.
David Paltiel at the Yale School of Public Health in New Haven, Connecticut, and his colleagues modelled the effect of a variety of testing strategies on the number of infections that would arise among 5,000 students during an 80-day semester (A. D. Paltiel et al. Preprint at medRxiv http://doi.org/d3cc; 2020).
In one scenario, the researchers assumed that five new cases would be imported each week, each infected student would infect 2.5 others and those who tested positive would be isolated. The team found that testing students every two days with a rapid and relatively cheap test would keep infections to around 135 over the semester, and cost US$470 per student per term. However, testing only weekly would result in an explosive growth in infections.
If the transmission rate were higher, keeping infections manageable would require daily testing, which would double the costs. The authors stress that preventive measures such as social distancing will therefore remain essential. The findings have not yet been peer reviewed.
8 July — One nation shows wildly disparate local infection rates
Europe’s largest effort to identify people who have been infected by the new coronavirus has found that roughly one-third of them did not show symptoms.
Between 27 April and 11 May, Marina Pollán at the Institute of Health Carlos III in Madrid and her colleagues tested more than 61,000 people from randomly selected households across Spain for SARS-CoV-2 antibodies, which are produced by the body’s immune system in response to coronavirus infection (M. Pollán et al. Lancet http://doi.org/gg332t; 2020). The study reported large geographical variations in the prevalence of antibodies: more than 10% of people in central areas such as Madrid tested positive, compared with less than 3% in most coastal provinces.
Nationwide, some 5% of people tested positive, of which around one in three were asymptomatic. On the basis of their results, the researchers estimate that roughly one million people previously infected with the coronavirus could have gone undetected in Spain because they did not show symptoms.
7 July — Autopsies links immune response to death from COVID-19
An autopsy-based study of 11 people who died from COVID-19 shows a mismatch between viral hotspots in the body and sites of inflammation and organ damage, suggesting that immune responses, rather than the virus itself, are responsible for death.
Numerous studies have suggested that the immune system contributes to the organ damage seen in some severe cases of COVID-19. Christopher Lucas and David Dorward at the University of Edinburgh, UK, and their colleagues conducted detailed autopsies to map signs of SARS-CoV-2 in the body, along with sites of inflammation and injury (D. A. Dorward et al. Preprint at medRxiv http://doi.org/d27t; 2020).
The survey of 37 anatomical sites, including the lungs, found little correlation between levels of the virus and inflammation: some tissues harboured the virus but were not inflamed, whereas others were damaged but did not contain high levels of SARS-CoV-2. The findings have not yet been peer reviewed.
26 June — Test frequency matters more than test sensitivity for stopping outbreaks
Communities such as universities where COVID-19 cases could quickly spiral out of control should frequently test large numbers of people for the new coronavirus — even if that means using a relatively insensitive test.
Tests that rely on the technique quantitative polymerase chain reaction (qPCR) can detect the merest traces of SARS-CoV-2 genetic material but are expensive and slow to return results. To gauge the importance of test sensitivity, Michael Mina at the Harvard T. H. Chan School of Public Health in Boston, Massachusetts, and his colleagues modelled the effect of widespread testing on viral spread in a large group of people (D. B. Larremore et al. Preprint at medRxiv, http://doi.org/d2gt; 2020).
The researchers found that weekly surveillance testing, paired with case isolation, would limit an outbreak even if the testing method was less sensitive than qPCR. By contrast, surveillance testing done every 14 days would allow the total number of infections to climb almost as high as if there were no testing at all. The findings have not yet been peer reviewed.
24 June — A finely detailed map reveals a viral protein’s Achilles heel
Scientists have created and described more than 3,800 variations of the protein that the new coronavirus uses to latch on to its targets — a feat that reveals which parts of the protein are crucial for binding to human cells.
Before SARS-CoV-2 invades a cell, a viral protein called spike fastens tightly to a receptor that sits on the surface of many human cells. Jesse Bloom at the Fred Hutchinson Cancer Research Center in Seattle, Washington, and his colleagues altered a single amino acid at a time in a key segment of spike to produce 3,804 variants of the protein (T. N. Starr et al. Preprint at bioRxiv http://doi.org/dz8r; 2020). Tests showed that many of these variants bind to the receptor at least as well as the protein in the coronavirus causing the current pandemic.
The tests allowed the team to pinpoint the amino acids that, if altered, impair the spike protein’s binding ability. This knowledge could help researchers to develop molecules that neutralize the virus’s ability to infect cells. The findings have not yet been peer reviewed.
23 June — A striking share of infected people never show classic symptoms
Less than one-third of people infected with SARS-CoV-2 fell ill with respiratory symptoms or fever, according to a survey of thousands of people in Italy.
More than 16,000 people in Lombardy have died of COVID-19, making the region the epicentre of Italy’s coronavirus outbreak. Piero Poletti at the Bruno Kessler Foundation in Trento, Italy, Marcello Tirani at the Health Protection Agency of Pavia in Italy and their colleagues studied people in Lombardy who had had close contact with an infected person.
Roughly half of these 5,484 contacts became infected themselves (P. Poletti et al. Preprint at https://arxiv.org/abs/2006.08471; 2020). Of those, 31% developed respiratory symptoms — such as a cough — or a fever; only 26% of those under the age of 60 did so. As a person’s age increased, so did their odds of experiencing symptoms and becoming ill enough to require intensive care, or to die. The results could inform hospitals’ outbreak preparations, the authors say.
The findings have not yet been peer reviewed.
22 June — CRISPR pinpoints host genes that aid viral invasion
A trawl through a monkey genome using the CRISPR–Cas9 genome-editing system has identified a handful of genes that might help the new coronavirus to infect its hosts.
The discovery of host genes that aid viral activity could aid the development of new therapies, and reveal why some people are more susceptible to COVID-19 than others. John Doench at the Broad Institute of MIT and Harvard in Cambridge, Massachusetts, Craig Wilen at Yale School of Medicine in New Haven, Connecticut, and their colleagues used CRISPR–Cas9 to alter genes in cultured monkey cells. They then looked for those genes that influenced viral infection and host-cell death (J. Wei et al. Preprint at bioRxiv http://doi.org/dzz3; 2020).
The team’s survey found genes that code for several proteins not known to assist the coronavirus. Among them are proteins in the TGF-β signalling pathway, which is involved in cell growth and death. Chemicals that inhibit this pathway also prevented coronavirus-induced cell death. The findings have not yet been peer reviewed.
19 June — Youth is a shield against infection by close contacts
People under the age of 20 are much less likely than their elders to catch the new coronavirus from an infected household member.
Yang Yang at the University of Florida, Gainesville, Zhi-Cong Yang at the Guangzhou Center for Disease Control and Prevention in China and their colleagues analysed viral transmission between infected people in Guangzhou and those who’d had close contact with them (Q. Jing et al. Lancet Inf. Dis. http://doi.org/dznw; 2020). After public-health officials had instituted isolation of infected individuals and quarantine of their contacts, people under the age of 20 had a 5.2% risk of being infected by a member of their household, compared with a 14.8% risk for people aged 20–59 and an 18.4% risk for people aged 60 and above.
The researchers also found that people with COVID-19 were at least as infectious before their symptoms started as after. The authors suggest that viral spread within households could be limited by providing facilities where infected people could isolate themselves from their families.
17 June — More than one billion people face increased risk of severe COVID-19
A host of common health problems boost a person’s risk of becoming seriously ill if infected by the new coronavirus. Now an analysis reveals the extent of this vulnerable group: more than 20% of the world’s population has at least one underlying condition that raises the risk of severe disease.
Andrew Clark at the London School of Hygiene & Tropical Medicine and his colleagues examined the prevalence of diabetes, cardiovascular problems and other conditions that predispose people infected with SARS-CoV-2 to severe COVID-19 (A. Clark et al. Lancet Glob. Health http://doi.org/dzk9; 2020). Analysing data from 188 nations, the team estimates that 1.7 billion people worldwide have an elevated risk of ‘severe’ illness. The researchers also estimate that nearly 350 million people — some of whom do not have underlying conditions — would require hospitalization if infected.
These findings can be used to assess how many high-risk people will need a vaccine once it is developed, the authors say.
16 June — Swiss survey finds that children are less susceptible to infection
Children and the elderly are less likely than adults under the age of 65 to show evidence of past SARS-CoV-2 infection, according to a survey of people in Geneva, Switzerland.
Silvia Stringhini at Geneva University Hospitals and her colleagues tested some 2,700 people aged 5 and older for antibodies produced by the immune system to prevent reinfection with the new coronavirus (S. Stringhini et al. Lancet http://doi.org/dzh5; 2020).
The researchers found that only one out of the 123 children aged 5–9 tested positive, although 21 of them lived with someone who had COVID-19 antibodies. Of 369 participants aged 65 or older, 11 lived with another person with COVID-19 antibodies and 15 tested positive.
The researchers say that the low prevalence for children suggests that they might be less susceptible to infection, whereas the low prevalence in the elderly might stem from less exposure to the virus and an ageing immune response.
15 June — Bars, karaoke and gyms can aid ‘superspread’
Clusters of coronavirus infections are often linked to events many people breathe heavily while packed together, such as karaoke parties and and gym sessions, according to a survey in Japan.
Hitoshi Oshitani at Tohoku University in Sendai, Japan, and his colleagues analysed clusters of at least five infected people who had all attended the same event or venue (Y. Furuse et al. Emerg. Inf. Dis. http://doi.org/ggz2hg; 2020). Many of the 61 ‘superspreading’ incidents they identified occurred in hospitals, nursing homes and other care facilities, but a little more than half took place at venues such as musical events, restaurants and workplaces.
One concert, for example, was the source of infection for more than 30 people, including performers, audience members and staff.
The team identified the probable founders of 22 of the superspreading events, and the timing for 16 of them. The results showed that half of the superspreading individuals were under the age of 40, and 41% had had no symptoms when they transmitted the virus.
12 June — Modified mice could aid the quest for vaccines and drugs
Two teams have developed a short cut to generating COVID-19 mouse models: using a harmless virus to make the rodents cells susceptible to infection.
The SARS-CoV-2 virus invades a human cell by attaching to receptors, including one called ACE2, on its surface. Mice have a different version of ACE2, making them impervious to SARS-CoV-2 infection. Transgenic mice carrying the human version of ACE2 are susceptible to infection but are scarce.
To develop a more widely available mouse model, a team led by Michael Diamond at Washington University in St. Louis, Missouri, and another by Jincun Zhao at the First Affiliated Hospital of Guangzhou Medical University in China used adenoviruses — a workhorse of gene therapy — to deliver the human ACE2 gene to the lung cells of mice. After exposure to SARS-CoV-2, these mice lost weight and developed pneumonia.
Diamond’s team successfully treated the ill mice with therapeutic antibodies (A. O. Hassan et al. Cell http://doi.org/dzbk; 2020). Zhao’s group used the mice to test an experimental vaccine and several therapies for COVID-19 (J. Sun et al. Cell http://doi.org/dzbm; 2020).
11 June — A massive number of viral imports seeded the UK outbreak
The new coronavirus has jumped into the United Kingdom more than 1,300 times — mostly from France and Spain, despite early headlines focusing on infected travellers from China and other parts of Asia.
COVID-19 has killed more than 40,000 people in the United Kingdom. To understand the origins of the outbreak there, a team led by Oliver Pybus at the University of Oxford, UK, and Andrew Rambaut at the University of Edinburgh, UK, analysed nearly 30,000 SARS-CoV-2 genomes (O. Pybus et al. Preprint at Virological https://go.nature.com/37ieyvw; 2020).
The team tracked the number of times the virus reached the United Kingdom and began to spread inside the country’s borders. Genomic analysis found that there were 1,356 such introductions, although the researchers say that this number is preliminary and probably an underestimate.
Travellers from Spain accounted for roughly one-third of those introductions, and travellers from France slightly less than one-third. People coming from China accounted for less than 0.1% of introductions. The findings have not yet been peer reviewed.
11 June — Virus conscripts a pair of human proteins to invade cells
Researchers have found a second protein that SARS-CoV-2 uses to enter human cells, potentially offering a new target for vaccines and drugs.
The SARS-CoV-2 protein called Spike is known to attach to a human protein called ACE2, which allows the virus to enter cells. Two teams of researchers have now found that the human protein neuropilin-1 (NRP1) also aids viral invasion.
Peter Cullen and Yohei Yamauchi at the University of Bristol, UK, and their colleagues showed that a fragment of the Spike protein can bind to NRP1 (L. Cantuti-Castelvetri et al. Preprint at bioRxiv http://doi.org/dx5c; 2020). Both this team’s findings and those of Mikael Simons at the Technical University of Munich, Germany, and his colleagues (J. L. Daly et al. Preprint at bioRxiv http://doi.org/dx5d; 2020) show that an antibody that binds to NRP1 can block infection of human cells grown in the laboratory.
The Simons team also found that in mice, NRP1 assists the entry of virus-sized particles into the central nervous system. The studies suggest that blocking the interaction between the virus and NRP1 could provide a way to combat coronavirus infection.
Neither study has been peer reviewed yet.
9 June — People who feel fine can unknowingly spread the virus
A massive coronavirus testing campaign in Vietnam has found evidence that infected people who never show any symptoms can pass the virus to others.
Early in the global COVID-19 outbreak, Vietnam began to repeatedly test people at high risk of infection. Those who tested positive were admitted to a hospital until they either recovered or tested negative.
Of roughly 14,000 people tested between mid-March and early April, 49 were infected. Le Van Tan at the Oxford University Clinical Research Unit in Ho Chi Minh City, Vietnam, and his colleagues monitored 30 of the 49 individuals and found that 13 developed no symptoms during their hospital stay (N. V. V. Chau et al. Clin. Infect. Dis. http://doi.org/ggzfz9; 2020).
Nasal swabbing showed that the infected but asymptomatic study participants had lower levels of viral RNA than infected people who felt ill at some point. But it’s “highly likely” that two of the asymptomatic participants were the source of infection for at least two other people, the authors say.
8 June— Lockdowns are a powerful tool against the pandemic
Lockdowns and other distancing measures have had resounding success at thwarting the new coronavirus, according to two independently conducted studies that examined different countries and measures of effectiveness.
Samir Bhatt at Imperial College London and his colleagues used data on COVID-19-related deaths to model viral transmission in 11 European countries (S. Flaxman et al. Nature http://doi.org/dxxs; 2020). The team found that in those nations, the combination of policies aimed at slowing the virus’s spread prevented more than 3 million deaths from the epidemic’s start to early May.
In each country, the actions taken were enough to halt the epidemic. Lockdowns — stay-at-home orders and policies that restrict face-to-face contact — were especially effective, reducing transmission by 81%.
Solomon Hsiang at the University of California, Berkeley, and his colleagues analysed how the growth rate of infections changed over time in China, the United States and four more countries that applied policies to prevent viral spread (S. Hsiang et al. Nature http://doi.org/dxxt; 2020). The analysis showed that across all 6 countries, anti-transmission measures averted roughly 500 million infections.
This team also found that lockdowns — policies that require people to stay at home whether or not they are infected — are effective at stemming viral spread.
5 June — Surfaces could pose only a modest risk for household spread
Contaminated surfaces might have only a minor role in transmitting COVID-19 within households.
Ricarda Schmithausen at the University of Bonn in Germany and her colleagues looked for traces of the virus SARS-CoV-2 in 21 households that each included at least one infected person (M. Döhla et al. Preprint at medRxiv http://doi.org/dxqn; 2020). The team found viral RNA in just 3% of samples from the most frequently touched objects, such as door knobs, and in 15% of samples taken from bathroom drains and toilets. The team could not grow infectious virus from any of the samples.
All 15 samples from air monitors designed to pick up fine respiratory aerosol particles tested negative for viral RNA, although the authors say that the method they used means this result should be interpreted cautiously.
The findings suggest that direct transmission of the coronavirus, for example through exhaled or coughed droplets, is probably the main route of infection. However, transmission in wastewater is a possible route of infection, the authors add.
The results have not yet been peer reviewed.
4 June — Blood type might influence COVID-19 risk
Researchers have identified two human gene variants that could make people more susceptible to lung failure associated with COVID-19.
Tom Karlsen at Oslo University Hospital and his colleagues analysed the genomes of roughly 4,000 people from Italy and Spain: 1,980 people with COVID-19 who developed respiratory failure and more than 2,000 people who did not have the disease (D. Ellinghaus et al. Preprint at medRxiv http://doi.org/dxk7; 2020). Those with severe COVID-19 were more likely to carry either of two gene variants than people without the disease.
One variant lies in the swathe of the genome that determines blood groups. A follow-up analysis found that people with blood type A+ had an increased risk of lung failure compared with those with other blood types, whereas those with type O blood were protected to some extent. The study flagged a second variant, on chromosome 3, that is near six genes, including one that interacts with the molecular receptor the virus uses to enter human cells.
The study has not yet been peer reviewed.
3 June — Drug hailed for its potency fails to prevent infection
A large clinical trial has found no evidence that the drug hydroxychloroquine protects people from COVID-19.
Some world leaders have embraced hydroxychloroquine as a treatment for COVID-19 or as an agent to prevent the disease. David Boulware at the University of Minnesota in Minneapolis and his colleagues randomly assigned 821 people to take either hydroxychloroquine or a placebo within 4 days of exposure to SARS-CoV-2 (D. R. Boulware et al. N. Engl. J. Med. http://doi.org/dxkv; 2020). Some study participants were health-care workers who had contact with infected people; others shared a house with an infected person.
About 12% of people given hydroxychloroquine developed COVID-19 within 2 weeks, compared with about 14% who were given the placebo. That difference is not statistically significant. Those taking the drug also reported more side effects than those taking the placebo.
The authors note an important caveat to the study: tests were not available for people, including health-care workers, unless they had symptoms of COVID-19. Therefore, asymptomatic cases are unaccounted for.
2 June — Could antibody tests downplay virus’s prevalence?
Antibody studies might underestimate the share of a population that has been infected with SARS-CoV-2.
In response to a pathogen attack, immune cells produce molecules called antibodies, which can linger in the blood and provide a record of infection. Isabel Rodríguez-Barraquer at the University of California, San Francisco, and her colleagues identify a potential source of bias in tests that detect the presence of antibodies against the new coronavirus (S. Takahashi et al. Preprint at OSF Preprints http://doi.org/dxc2; 2020).
Most antibody tests have been validated using blood samples from people hospitalized with severe disease. But these individuals, who make up only a small fraction of infected people, might have higher levels of antibodies circulating in their body than have people with mild or no symptoms.
The researchers say more detailed studies are needed to assess how well antibody tests detect previous infection in people who had mild disease.
The findings have not yet been peer reviewed.
1 June — Positive coronavirus test is no guarantee of infectiousness
People with COVID-19 are unlikely to spread the new coronavirus if more than eight days have passed since their symptoms began, according to experiments in monkey cells.
Jared Bullard at the University of Manitoba in Winnipeg, Canada, and his colleagues seeded cultured monkey cells with 90 human samples that had tested positive for SARS-CoV-2 RNA (J. Bullard et al. Clin. Infect. Dis. http://doi.org/dw8z; 2020). The researchers found that RNA-positive samples collected more than eight days after a person’s symptoms began did not infect the cells — suggesting that people who test positive for viral RNA are not necessarily infectious.
Hospital patients who still test positive for viral RNA weeks after they began feeling ill might not need to be strictly isolated, the team says.
29 May — The nose could be the body’s entry point to infection
The nose is the probable starting point for COVID-19 infections.
Richard Boucher and Ralph Baric at the University of North Carolina at Chapel Hill and their colleagues tracked the ease with which the new coronavirus infects various cell types in the respiratory tract. The researchers found a gradient of infectivity that decreases from the upper to the lower respiratory tract: the most easily infected cells are in the nasal cavity, and the least easily infected deep in the lungs. (Y. J. Hou et al. Cell http://doi.org/dw2j; 2020). That gradient mapped neatly onto the distribution of cells that express ACE2, a protein that SARS-CoV-2 uses to enter cells.
The authors speculate that the virus gets a foothold in the nose, then sneaks down the respiratory tract when breathed into the airways. They say the results support the use of masks and preventative measures such as nasal cleansing.
28 May — A lost opportunity to stop viral spread in the United States
Genomic analysis has contradicted a high-profile finding about the origins of the first community spread of the new coronavirus in the United States.
In late February, a widely publicized genomic analysis suggested that SARS-CoV-2 had been silently spreading for weeks in Washington state. The analysis traced the outbreak’s origin to a traveller designated WA1 — even though officials had quickly detected WA1’s infection after his arrival from China on 15 January and had done extensive contact tracing to stop transmission.
But modelling by Michael Worobey at the University of Arizona, Tucson, and his colleagues suggests that WA1 did not trigger a wider outbreak. Instead, the team found evidence that the virus that spread in Washington reached the state from China in mid-February (M. Worobey et al. Preprint at bioRxiv http://doi.org/dwx3; 2020). The findings have not yet been peer reviewed.
The four weeks between WA1’s arrival and the arrival of the actual source were a “missed opportunity” to stop the virus from taking hold in the United States, the authors say.
27 May — Superspread in Israel caused a high portion of infections
An “extremely high level” of viral superspread helped to seed the new coronavirus across Israel, according to the authors of a genomic analysis.
Adi Stern at Tel Aviv University in Israel and her colleagues sequenced and analysed more than 200 SARS-CoV-2 genomes from people across Israel (D. Miller et al. Preprint at medRxiv http://doi.org/dwvb; 2020). The results, which have not yet been peer reviewed, show that only 1–10% of infected people caused 80% of the next wave of cases, illustrating the power of superspreaders in viral transmission.
The analysis also found that travelers from the United States and Europe carried the virus to Israel, but US travelers were responsible for a disproportionate share of viral spread. One possible explanation: Israel began restricting entry of people arriving from Europe before it banned US arrivals.
26 May — Exposed children escape infection more often than adults
Children and adolescents under the age of 20 are much less likely than adults to become infected by the new coronavirus, finds a large systematic review of journal articles, preprints and reports.
Russell Viner at University College London and his colleagues screened more than 6,000 studies, of which 18 provided data that met the authors’ criteria for inclusion. The 18 included 7 that had been peer reviewed (R. M. Viner et al. Preprint at medRxiv http://doi.org/dwp6; 2020).
Studies that traced the contacts of infected individuals show that children are 56% less likely to get infected than adults when in contact with an infected person. The analysis suggests that children have played a smaller part than adults in spreading the virus in the population, but the evidence for this finding is weak. There has not been enough research to determine whether infected children are less likely than adults to pass on the infection, the authors conclude.
The study has not yet been peer reviewed.
25 May — Trump’s favoured drug shows no benefit — but another drug does
World leaders, including US President Donald Trump, have touted the antimalarial drug hydroxychloroquine as a treatment for COVID-19. But a study of nearly 100,000 people found no benefit to the drug and linked it instead to an elevated risk of death and abnormal heart rhythms.
Mandeep Mehra at the Brigham and Women’s Hospital Heart and Vascular Center in Boston, Massachusetts, and his colleagues analysed the health records of more than 96,000 people treated for COVID-19. The study drew on data from patients at 671 hospitals on 6 continents (M. R. Mehra et al. The Lancet http://doi.org/ggwzsb; 2020). Roughly 15% of these patients received hydroxychloroquine, the related drug chloroquine, or one or the other of these drugs paired with an antibiotic.
Compared with people who did not take the drugs, people in all four treatment arms were more likely to die in hospital and more likely to develop a disordered heartbeat, or arrhythmia. The authors say that only people enrolled in clinical trials should take the drugs.
A separate trial of the drug remdesivir showed that it shortens the recovery of people hospitalized with COVID-19. John Beigel at the National Institutes of Allergy and Infectious Diseases in Rockville, Maryland, and his colleagues studied more than 1,000 people enrolled in a randomized, double-blind trial and found that those who took remdesivir had a median recovery time of 11 days, compared with 15 days for those who took a placebo (J. H. Beigel et al. N. Engl. J. Med. http://doi.org/dwkd; 2020).
Editors’ note: The Lancet has published a retraction (https://www.thelancet.com/lancet/article/s0140673620313246) of the controversial paper by Mehra et al. on hydroxychloroquine.
22 May — DNA vaccines protect monkeys from coronavirus
Monkeys were protected from the new coronavirus after receiving a DNA vaccine against the virus.
Dan Barouch at Harvard Medical School in Boston, Massachusetts, and his colleagues explored vaccines composed of DNA (J. Yu et al. Science http://doi.org/dwfb; 2020). This type of vaccine prompts the recipient’s cells to make a pathogen or its components. That, in turn, stimulates the immune system.
The researchers developed six DNA vaccines based on a coronavirus protein called spike and tested them in rhesus macaques (Macaca mulatta). The animals mounted an antibody response similar to that seen in macaques and people who had recovered from SARS-CoV-2 infection.
The team then gave doses of coronavirus to the vaccinated monkeys, which developed only mild illness. Viral multiplication in the animals was generally lower than in unvaccinated monkeys, probably because the vaccinated animals’ immune systems kept the virus in check.
21 May — Potent human antibodies could inspire a vaccine
A vaccine typically works by triggering the body’s immune response, which generates antibodies that fend off a particular virus. But some viruses do not stimulate a protective antibody response, which means there’s no guarantee that a vaccine can be developed for every disease.
Davide Robbiani at Rockefeller University in New York City and his colleagues studied 68 people who had recovered from SARS-CoV-2 infection and found that they all had generated varying amounts of antibodies against the virus. A fraction of these antibodies strongly blocked the coronavirus from invading human cells (D. F. Robbiani et al. Preprint at bioRxiv http://doi.org/ggwfcm; 2020). The work has not yet been peer reviewed.
People who’d recovered from severe disease had higher levels of these potent antibodies, on average, than people whose illness was milder. But every participant appeared to be capable of making them. The authors suggest that a vaccine designed to elicit these potent antibodies might be universally effective.
21 May — Monkeys resist re-infection after recovering from the virus
Monkeys that had recovered from infection with the new coronavirus were protected from re-infection, although how long the protection lasts is unclear.
Public-health officials need to know whether people who have been infected with SARS-CoV-2 can be infected again. To address this issue, Dan Barouch at Harvard Medical School in Boston, Massachusetts, and his colleagues gave doses of the coronavirus to nine rhesus macaques (Macaca mulatta). The monkeys developed mild symptoms, such as appetite loss, as well as antibodies against the virus (A. Chandrashekar et al. Science http://doi.org/dwck; 2020).
Roughly one month later, the researches gave the monkeys another dose of virus. Over the following two weeks, the team detected low, rapidly declining levels of viral RNA in the animals’ noses and almost none in the monkeys’ lungs. All of the monkeys mounted an antibody response to the second dose of SARS-CoV-2, suggesting that their immune systems had fought off the virus.
20 May — The virus ravages organs from heart to brain
Autopsies have found the new coronavirus not only in the lungs, but also in the kidneys, heart, brain and other organs.
COVID-19 is principally considered a respiratory disease, but some infected people experience non-respiratory symptoms, such as stroke. Tobias Huber at the University Medical Center Hamburg-Eppendorf in Germany and his colleagues conducted autopsies on 27 people with COVID-19. They found that the virus was most abundant in the lungs, but was also present at lower levels in the kidneys, liver, heart, brain and blood (V. G. Puelles et al. N. Engl. J. Med. http://doi.org/dv56; 2020).
By scrutinizing databases of genetic activity, the team found that three genes known to encourage SARS-CoV-2 infection are highly active in kidney cells. Additional analysis of 6 people detected virus in all examined kidney compartments, which helps to explain the kidney damage seen in some people with the illness.
19 May — An antibody blocks the new coronavirus and an older relative
An antibody discovered in the blood of a person who survived SARS could help others to fight COVID-19.
The coronavirus that caused the 2003 SARS outbreak is a distant relative of SARS-CoV-2, the virus responsible for the current pandemic. The newfound antibody, dubbed S309, recognizes and blocks both viruses, report David Veesler at the University of Washington in Seattle, Davide Corti at Vir Biotechnology in Bellinzona, Switzerland, and their colleagues (D. Pinto et al. Nature https://doi.org/dv4x; 2020).
The antibody is an immune signalling molecule that attaches to a viral protein called spike, which both viruses use to enter human cells. The team’s structural analysis shows that S309 binds to a location on spike that is distinct from the attachment site of some of the person’s other coronavirus-targeted antibodies. Two cocktails, each combining one of these two antibodies with S309, were better at blocking the virus than was each antibody alone.
18 May — Dogs can catch coronavirus from their owners
The first two dogs reported to have coronavirus probably caught the infection from their owners, say researchers who studied the animals and members of the infected households in Hong Kong. An analysis showed that the viral genetic sequences from the dogs were identical to those from the infected people.
The researchers studied 15 dogs who lived with people with COVID-19 (T. H. C. Sit et al. Nature http://doi.org/dvt4; 2020). Only two — a Pomeranian and a German shepherd — caught the disease. The team detected viral RNA and antibodies in both dogs, and live virus in one. Neither dog became noticeably sick.
The study showed no evidence that dogs can pass the infection to other dogs or to people.
15 May — Promising vaccine shields monkeys from lung damage
An experimental COVID-19 vaccine protected monkeys from pneumonia and prompted a strong immune response in the animals.
Vincent Munster at the National Institute of Allergy and Infectious Diseases in Hamilton, Montana, Sarah Gilbert at the University of Oxford, UK, and their colleagues designed a vaccine that encodes the new coronavirus’s spike protein, which it uses to invade host cells (N. van Doremalen et al. Preprint at bioRxiv http://doi.org/dvvd; 2020). The researchers injected 6 rhesus macaques (Macaca mulatta) with the vaccine before giving the animals high doses of virus.
The vaccinated monkeys all developed neutralizing antibodies — which can prevent a virus from entering cells — against SARS-CoV-2. Vaccinated animals had much lower levels of viral RNA in their lung tissue than unvaccinated animals, suggesting that the vaccine stopped the virus from multiplying in the monkeys’ lungs. Two of the three control monkeys developed pneumonia; none of the vaccinated monkeys did. The research has not yet been peer reviewed.
A clinical trial of the vaccine is now under way.
15 May — Lifting lockdown could spell surge of infections for France
More than 20,000 people in France have died of COVID-19, but the nation’s infection rate in mid-May stood at roughly 5% — well short of the 65% needed for herd immunity.
Simon Cauchemez at the Pasteur Institute in Paris and his colleagues modelled France’s coronavirus outbreak. (H. Salje et al. Science, http://doi.org/dvt3; 2020). They found that France’s lockdown, which began 17 March, reduced viral spread by 77%. The team projected that by the time the lockdown was relaxed on 11 May, an estimated 4.4% of the population would have been infected.
Some two-thirds of the population would need to be immune for immunity alone to control the epidemic. As a result, herd immunity cannot prevent “a second wave at the end of the lockdown”, the authors write.
14 May — ‘Superspread’ at a choir practice infects dozens
A single ill person who attended a choir practice in Washington State led to the probable infection of more than 50 choir members, including 2 who died.
Lea Hamner and her colleagues at Skagit County Public Health in Mount Vernon, Washington, analysed numerous local cases of COVID-like illness and traced them to an evening choir practice on 10 March (L. Hamner et al. Morb. Mortal Wkly. Rep. 69, 606–610; 2020). One symptomatic person attended the 2.5-hour practice and later tested positive for SARS-CoV-2. Of the other 60 people in attendance, 32 became ill with confirmed COVID-19 and an additional 20 became ill with probable infections.
Choir members sat in close-packed rows and sang for long periods, which might have contributed to viral transmission. This superspreading event emphasizes the importance of avoiding crowds and close interactions to keep the virus at bay, the authors say.
14 May — The very youngest children are most likely to enter hospital
Children with COVID-19 are at a lower risk of death than are adults with the disease, according to the largest study of infected children in Europe.
Silvia Garazzino at the University of Turin, Italy, and her colleagues analysed data from children under the age of 18 who turned up at hospitals and clinics with COVID-19 symptoms. All 168 who tested positive for the coronavirus recovered fully (S. Garazzino et al. Preprint at Eurosurveillance http://doi.org/dvk8; 2020). The study has not yet been peer reviewed.
Nearly 80% of infants under the age of one were hospitalized, compared with 53% of those between the ages of 11 and 17. A national survey estimates that the overall hospitalization rate for infected children in Italy is much lower — around 4%.
Two-thirds of the children had at least one infected parent, whose symptoms often appeared before the child’s did.
13 May — New York City’s infection hotspots have high numbers of commuters
New York City neighbourhoods that were COVID-19 hotspots between March and May correlate with those that were home to the highest number of commuters over the past three months.
To understand why deaths and hospitalizations from COVID-19 varied so substantially between the city’s neighbourhoods, Stephen Kissler at the Harvard T.H. Chan School of Public Health in Boston, Massachusetts, and his colleagues compiled coronavirus test results from about 1,700 women who came to 6 city hospitals to give birth (S. M. Kissler et al. Preprint at https://bit.ly/2Aq7dpb; 2020).
The team analysed the postal codes of infected women to estimate disease prevalence in city neighbourhoods. The researchers then compared this information with location data from Facebook that revealed the number of daily trips that people take into and out of each neighbourhood, and found a link between a neighbourhood’s infection rate and the number of trips taken by its residents.
Many of the commuters are probably ‘essential workers’, who should be protected to prevent the virus’s spread, the authors say.
12 May — The body launches a sweeping antibody response to coronavirus
People infected by the new coronavirus make antibodies against several of the virus’s proteins — a finding that could lead to more effective vaccines and more sensitive tests to determine who has already been infected and might now be immune.
Niloufar Kavian and Sophie Valkenburg at the University of Hong Kong and their colleagues wanted to determine which SARS-CoV-2 proteins are targeted by immune molecules called antibodies, which help to fight infection.
The team found that 15 people with COVID-19 had more antibodies against 11 viral proteins than did healthy people before the pandemic (A. Hachim et al. Preprint at medRxiv http://doi.org/ggtrxh; 2020). Tests for antibodies against three of these proteins distinguished infected people from healthy controls.
Much of the effort to develop vaccines and diagnostic tests has focused on a viral protein called Spike. But these results, which have not yet been peer reviewed, suggest that other proteins might also be important determinants of immunity against SARS-CoV-2.
11 May — High risk of COVID-19 death for minority ethnic groups is a troubling mystery
People who are not white face a substantially higher risk of dying from COVID-19 than do white people — and pre-existing health conditions and socioeconomic factors explain only a small part of the higher risk.
In the most sweeping study of its kind, Ben Goldacre at the University of Oxford, UK, and his colleagues examined the medical records of more than 17 million residents of England (E. Williamson et al. Preprint at medRxiv http://doi.org/dt9z; 2020). The analysis, which has not yet been peer reviewed, showed that medical conditions such as diabetes are linked to a higher risk of death from the new coronavirus.
But the prevalence of such conditions in people who belong to minority ethnic groups plays only a small part in the heightened risk, as does the prevalence of social disadvantages such as low income. The researchers say that there is an urgent need for better measures to protect people in minority ethnic groups from the disease.
8 May — A strong antibody response is common in people who’ve recovered
Nearly everyone who recovers from COVID-19 makes antibodies against the new coronavirus, according to a study of more than 1,300 people who had symptoms of the disease.
Ania Wajnberg, Carlos Cordon-Cardo and their colleagues at the Icahn School of Medicine at Mount Sinai in New York City found that more than 99% of study participants who had been infected eventually developed antibodies — suggesting that they are immune from reinfection for an unknown length of time (A. Wajnberg et al. Preprint at medRxiv http://doi.org/dt5t; 2020). The immune response could be slow: some study volunteers didn’t produce detectable antibodies until one month after they first started feeling ill. The team found that a person’s age and sex didn’t affect their chance of developing antibodies.
Almost 20% of study volunteers tested positive for viral RNA two or more weeks after their symptoms ended. This might mean that the presence of viral RNA is not a good indicator of whether the body has cleared the virus. The study has not yet been peer reviewed.
7 May — Even laypeople could use this new test to detect the coronavirus
A test that uses a CRISPR gene-editing system can detect the new coronavirus in an hour, without the need for specialized equipment or trained personnel.
Feng Zhang at the Broad Institute of MIT and Harvard in Cambridge, Massachusetts, and his colleagues sought to develop a test for SARS-CoV-2 that would be quicker and simpler than the current procedure, which requires expensive lab equipment and scarce reagents. The team’s CRISPR-based protocol can be performed by a layperson with access to a sous vide cooker, a piece of kitchen equipment that is commonly available for less than US$40 (J. Joung et al. Preprint at https://go.nature.com/35csgqk; 2020). The test makes results available on paper strips similar to those used in pregnancy tests. The results have not yet been peer reviewed.
The team says the test could be used in doctors’ offices, workplaces and other settings where fast diagnosis is necessary.
6 May — Speedy technique churns out synthetic viruses
Researchers have used yeast cells to create a synthetic version of the SARS-CoV-2 genome much more quickly than other methods can achieve.
The SARS-CoV-2 genome is composed of RNA, but the protocol developed by Joerg Jores and Volker Thiel at the University of Bern in Switzerland and their colleagues uses a dozen overlapping stretches of the SARS-CoV-2 genome converted into DNA (T. T. N. Thao et al. Nature http://doi.org/ggttcr; 2020). The team inserted these DNA fragments into cells of the yeast Saccharomyces cerevisiae, which stitched them into a complete viral genome.
The team then built live viruses by converting the synthetic genome back into RNA and inserting these strands into human cells. The synthetic coronaviruses took a week to make. The technique could be used to assemble viruses rapidly to study the biological effects of new mutations, the researchers say.
5 May — What stopped the epidemic in China? Two teams show there is no easy answer
New evidence shows the value of school closures, travel bans and other painful measures in curbing the coronavirus epidemic in China.
Marco Ajelli at the Bruno Kessler Foundation in Trento, Italy, Hongjie Yu at Fudan University in Shanghai and their colleagues found that after authorities mandated a stringent lockdown, people in Shanghai and Wuhan cut their encounters with others from 15–20 per day to roughly 2 per day (J. Zhang et al. Science http://doi.org/ggthtr; 2020). This drastic social distancing was enough to bring the epidemic under control in the two cities.
The team’s modelling work suggests that, in Shanghai, school closures alone would not have stopped the epidemic — but did lower the number of new infections per day at the epidemic’s peak, which relieved stress on hospitals.
Another study, by Shengjie Lai at the University of Southampton, UK, and his colleagues, shows that quick detection of infections and isolation of infected people were the most effective steps for containing COVID-19 cases in China (S. Lai et al. Nature http://doi.org/dtr4; 2020). But even with those efforts in place, the number of cases would have soared if officials hadn’t restricted travel and social interactions, as well.
If epidemic-control actions had been delayed by only three weeks, the number of infected people in China might have been 18 times higher, the authors found.
4 May — Portraits of a viral enzyme could aid hunt for drugs
Molecular snapshots of a key SARS-CoV-2 enzyme in action provide clues to how drugs, including the experimental therapy remdesivir, attack the virus.
Remdesivir has been shown in an early trial to speed up the recovery of people with COVID-19. The compound blocks the action of a viral enzyme called an RNA-dependent RNA polymerase.
A team led by Patrick Cramer at the Max Planck Institute for Biophysical Chemistry in Göttingen, Germany, used an imaging technique called cryo-electron microscopy to map the 3D shape of the enzyme as it copied the virus’s genetic material (H. S. Hillen et al. Preprint at bioRxiv http://doi.org/dtgw; 2020; not peer reviewed before posting). The researchers say that further studies of the polymerase could lead to the identification of new antiviral compounds.
A separate team led by Eric Xu at the Shanghai Institute of Materia Medica in China solved the structure of the polymerase while it was linked to an RNA snippet incorporating a molecule of remdesivir (W. Yin et al. Science http://doi.org/dtnb; 2020). The results could help researchers to design powerful drugs that block the polymerase’s activity, the authors say.
1 May — Immune system shows abnormal response to COVID-19
The immune response to SARS-CoV-2 differs from the response prompted by other respiratory viruses, according to an analysis of infected cells, ferrets and people. The finding supports the idea that treatments targeting the immune system could help people with COVID-19.
Benjamin tenOever at the Icahn School of Medicine at Mount Sinai in New York City and his colleagues found that cells infected with SARS-CoV-2 produce unusually low levels of antiviral proteins called interferons compared with cells infected with other respiratory viruses (D. Blanco-Melo et al. Cell https://go.nature.com/3bWE82b, 2020). But levels of some proteins, such as IL-6, that activate more general immune responses are higher in infected ferrets and people than in uninfected controls.
The results suggest an immune imbalance: low levels of interferons reduce a cell’s ability to limit viral replication, and the activation of less-specific immune responses promotes inflammation.
30 April — Young children are not immune to COVID-19
Children are as likely as adults to become infected with SARS-CoV-2 after close contact with an infected person, according to a study of people in Shenzhen, China.
Justin Lessler at Johns Hopkins Bloomberg School of Public Health in Baltimore, Maryland, Tiejian Feng at the Shenzhen Center for Disease Control and Prevention and their colleagues analysed nearly 400 cases of COVID-19 and 1,300 people who were ‘close contacts’ of the infected people (Q. Bi et al. Lancet Inf. Dis. http://doi.org/dtd7; 2020). The team found that 7% of close contacts younger than age 10 became infected — roughly the same as in the population overall. The work was first posted online as a preprint 27 March (http://doi.org/dpf9).
The researchers also found that just 9% of original cases were responsible for 80% of infections detected in close contacts. Such ‘superspreading’ events could lead to “large COVID-19 clusters”, the authors write.
29 April — SARS-CoV-2 might invade by hijacking its host’s immune defences
The new coronavirus invades human cells after one of its proteins binds with ACE2, a protein found in cells in many human organs. But little has been known about that crucial interaction.
To learn more, Alex Shalek at Harvard Medical School and the Massachusetts Institute of Technology (MIT) in Boston, Jose Ordovas-Montanes at the Broad Institute of MIT and Harvard in Cambridge, Massachusetts, and their colleagues studied airway cells from people with influenza (C. G. K. Ziegler et al. Cell http://doi.org/ds9j; 2020). Both influenza virus and SARS-CoV-2 invade the respiratory tract.
The team found that in people with flu, signalling molecules called interferons — which normally help to fend off viruses — switch on the host genes encoding the ACE2 protein. The result suggests that the body’s defences against viral attack drive the activation of the gene for ACE2.
28 April — ‘Dry swabbing’ offers a workaround to test-chemical scarcity
Wide-scale genetic testing for SARS-CoV-2 has been hampered, in part, by shortages of the solutions used to store sampling swabs and extract viral RNA from them. To overcome this difficulty, a team led by Lea Starita and Jay Shendure at the University of Washington in Seattle developed a procedure for detecting viral RNA in swabs without the highly sought solutions (S. Srivatsan et al. Preprint at bioRxiv http://doi.org/ds6k; 2020; not peer reviewed before posting).
The ‘dry swab, extraction-free’ procedure correctly detected viral RNA in 9 out of 11 samples from people known to have SARS-CoV-2 infections. Conventional extraction methods yielded positive results in only 8 of the 11. The researchers say that their protocol could enable a massive scale-up in the use of self-collected samples for genetic testing at centralized laboratories.
27 April — Hospital toilets can be a hotspot for airborne viral RNA
The new coronavirus’s RNA can travel through the air, and might spread by way of small particles exhaled by infected people.
Ke Lan at Wuhan University in China and his colleagues tested the concentration of SARS-CoV-2 RNA in aerosols — fine airborne particles — at two hospitals treating people with COVID-19 (Y. Liu et al. Nature https://doi.org/10.1038/s41586-020-2271-3; 2020).
The team detected elevated levels of viral RNA in locations such as a small toilet used by patients, and staff changing rooms. No viral RNA was detected in staff rooms after they had been disinfected. Low to undetectable levels were found in the hospitals’ well-ventilated patient wards.
The presence of airborne viral RNA suggests that SARS-CoV-2 has the potential to spread by way of aerosols, the researchers say. They suggest that measures such as routine disinfection and better ventilation could help to control the virus’s spread.
24 April — Spit could be the solution to testing shortages
A person’s saliva accurately reveals whether they are infected with SARS-CoV-2, a finding that could make tests for the virus safer and more widely available.
The gold-standard test for coronavirus infection requires a long swab to be rubbed against the back of the throat. But such swabs are in short supply, and swabbing can prompt people to cough or sneeze, potentially launching a barrage of viral particles.
Anne Wyllie at the Yale School of Public Health in New Haven, Connecticut, and her colleagues collected both saliva and throat samples from people hospitalized with COVID-19 (A. Wyllie et al. Preprint at medRxiv, http://doi.org/ggssqf, 2020; not peer reviewed before posting). The team’s testing did not detect the virus in some patients’ throat-swab samples — but did detect it in the same patients’ saliva samples. Saliva testing also showed that two health-care workers who felt fine and had negative throat tests were actually infected.
23 April — Intensive testing finds a small town’s many silent infections
A large proportion of people with COVID-19 have no symptoms, according to research in a small Italian town.
On 21 February, the town of Vo’ reported Italy’s first COVID-19 death, leading authorities to ban movement in the town and end public services and commercial activities there for two weeks. Andrea Crisanti at Imperial College London and his colleagues swabbed almost every resident of Vo’ for viral RNA at the beginning and end of the lockdown.
The team found that some 43% of the people infected with SARS-CoV-2 in the town reported no fever or other symptoms (E. Lavezzo et al. Preprint at medRxiv http://doi.org/ggsmcj; 2020; not peer reviewed before posting). The researchers observed no statistically significant difference in potential infectiousness between those who reported symptoms and those who did not.
Asymptomatic and pre-symptomatic individuals have a key role in COVID-19 transmission, which makes it difficult to control the disease without strict social distancing, the authors say.
22 April — A vaccine candidate shows early success in an animal trial
An experimental vaccine protects monkeys from infection with the virus that causes COVID-19.
A team led by Chuan Qin at the Peking Union Medical College in Beijing injected rhesus macaques (Macaca mulatta) with three doses of a vaccine comprised of chemically inactivated particles of SARS-CoV-2 (Q. Gao et al. Preprint at bioRxiv http://doi.org/dskt; 2020; not peer reviewed before posting). Eight monkeys were then intentionally exposed to the virus.
All four monkeys given a high dose of the vaccine had no detectable virus in their throat or lungs seven days after exposure. Monkeys that received a lower dose of vaccine showed some signs of coronavirus infection — but their levels of virus were much lower than in exposed animals that received no vaccine. This month, the company developing the vaccine received approval to start human safety trials on it.
20 April — How Hong Kong stemmed viral spread without harsh restrictions
Hong Kong slowed the spread of SARS-CoV-2 through a combination of intensive surveillance, quarantining and social distancing without relying on severe measures used elsewhere.
In January, the authorities in Wuhan, where the coronavirus outbreak began, halted travel out of the city in an attempt to control the spread of the virus that causes COVID-19. But Hong Kong relied on a programme that included widespread testing, quarantining of those who had been in contact with infected people, and distancing measures such as school closures. When Peng Wu at the University of Hong Kong and her colleagues surveyed residents in early March, 99% said they wore a mask in public and 85% said they avoided crowds (B. J. Cowling et al. Lancet Public Health http://doi.org/dsfw; 2020).
The combination of public behavioural changes and government measures kept the virus’s spread relatively low in Hong Kong during the period to the end of March, the team found.
17 April — Vaccine from viral spikes holds promise
A key portion of a coronavirus protein could form the basis of a safe and effective vaccine.
Coronavirus particles bristle with spiny ‘spike proteins’. A portion of the spike called the receptor-binding domain recognizes and attaches to a molecule found on the surface of many human cells, allowing the viral particle to gain entry into those cells.
Hyeryun Choe and Michael Farzan at the Scripps Research Institute in Jupiter, Florida, and their colleagues immunized rats with fragments of the spike’s binding domain (B. D. Quinlan et al. Preprint at bioRxiv, http://doi.org/ggrs5t; 2020; not peer reviewed before posting). In response, the rodents’ immune systems made antibodies that can recognize coronavirus and prevent it from infecting cells.
Further experiments suggested that these antibodies are unlikely to make host cells more susceptible to coronavirus infection — one of the main safety concerns for vaccines.
16 April — Ski buffs helped to seed coronavirus in Iceland
Holidaymakers returning from ski trips to the Alps helped to bring the coronavirus to Iceland.
In late January, Kari Stefansson at deCODE Genetics-Amgen in Reykjavik and his colleagues began testing for SARS-CoV-2 among Iceland residents at high risk of exposure to the virus, such as travellers to China (D. F. Gudbjartsson et al. N. Engl. J. Med. http://doi.org/ggr6wx; 2020). Some 13% of the 9,199 people tested by early April were infected. The team sequenced viral RNA from people who tested positive and found that some of the strains had probably originated in Austria or Italy, which both have Alpine ski resorts.
Tests in the second half of March on more than 2,000 randomly selected individuals found that only 0.6% were infected. The researchers say their analysis suggests that measures to contain the virus through testing, contact tracing and quarantining have been successful in Iceland.
15 April — Relief from social distancing could unleash the virus anew
Cases of COVID-19 are likely to surge after current social-distancing measures are eased, according to models.
Yonatan Grad, Marc Lipsitch and their colleagues at the Harvard T.H. Chan School of Public Health in Boston, Massachusetts, modelled the spread of coronaviruses in places that have temperate climates, such as the United States. The results helped the team to predict the spread of SARS-CoV-2, the coronavirus that causes COVID-19 (S.M. Kissler et al. Science http://doi.org/drz3; 2020).
The researchers found that if SARS-CoV-2 spreads more efficiently in some seasons than in others — as influenza virus does, for example — the peak number of COVID-19 cases after social distancing ends could be larger than the peak number without any social distancing at all. That’s because distancing measures leave a high proportion of people susceptible to infection, leading to a spike of disease if viral transmission ramps up late in the year.
If human immunity to SARS-CoV-2 wanes over the course of a few years, the virus is likely to cause repeated outbreaks in wintertime, the authors say.
15 April — Common sequencing technique could speed large-scale diagnosis
A standard genomic-analysis method that can sequence tens of thousands of DNA samples in a day has been adapted to detect the virus that causes COVID-19.
In a testing protocol proposed by Jonathan Schmid-Burgk at the Broad Institute of MIT and Harvard in Cambridge, Massachusetts, and his team, every sample being tested for SARS-CoV-2 would be tagged with a unique DNA sequence that would serve as a biological barcode (J. L. Schmid-Burgk et al. Preprint at bioRxiv, http://doi.org/drzc; 2020; not peer reviewed before posting). High-speed sequencing instruments common in research laboratories around the world could then be used to analyse as many as 100,000 DNA samples at one time.
The authors anticipate that if clinical testing validates the method, then millions of samples could be analysed per day at each sequencing site — a far more efficient output than that of current testing techniques.
Correction: An earlier version did not include the name of the paper’s corresponding author, Jonathan Schmid-Burgk.
10 April — A viral enzyme’s structure points to possible drugs
Scientists have detailed the crystal structure of one of SARS-CoV-2’s key proteins, an enzyme called a protease that the virus needs to replicate within our cells.
Hualiang Jiang, Zihe Rao and Haitao Yang at ShanghaiTech University in China and their colleagues deposited the structure in a protein data bank two months ago, and have since used it to help them identify compounds that inhibit the protease (Z. Jin et al. Nature https://doi.org/10.1038/s41586-020-2223-y; 2020). The team’s screening revealed several powerful viral inhibitors, including ebselen, whose safety has already been tested in people.
These inhibitors work by infiltrating a hollow in the protease. Proteases found in other coronaviruses have a similar hollow, raising hopes that a single compound might help to treat a wide variety of diseases caused by coronaviruses.
9 April — Absent antibodies suggest mystery immune response
After recovering from infection with SARS-Cov-2, many people have high levels of antibodies against the virus. But a recent study finds that in some recovered patients, such antibodies are present at very low levels — and in some cases are undetectable.
When a foreign microbe intrudes on the body, the immune system usually makes proteins called antibodies that help to fight off the invader. A team led by Jinghe Huang and Fan Wu at Fudan University in Shanghai, China measured antibodies to the novel coronavirus in 175 volunteers who had recovered from mild infections (F. Wu et al. http://doi.org/dxb9, 2020; not peer reviewed before posting). About 30% of the volunteers — and especially those under the age of 40 — never developed high levels of SARS-CoV-2 antibodies, suggesting that other immune responses helped rid them of their infections.
8 April — Viral load soars as infected people start feeling ill
Viral RNA levels are highest in people with COVID-19 soon after their symptoms appear, according to two separate research teams.
Kwok-Yung Yuen at The University of Hong Kong–Shenzhen Hospital, China, and his colleagues analysed saliva samples coughed up by 23 people infected with SARS-CoV-2. The team found that study participants’ viral concentrations peaked shortly after they started feeling ill, and began declining about one week after the peak.
The more viral RNA detected in a person’s body, the more they excrete when coughing or sneezing. The authors say that the high levels of SARS-CoV-2 particles detected at the onset of symptoms suggest that the virus can be transmitted easily between people, even when symptoms are relatively mild (K. K.-W. To et al. Lancet Infect. Dis. http://doi.org/ggp4qx; 2020).
The results are consistent with another study of nose and throat swabs from 18 people with COVID-19. The concentrations of viral RNA in the 17 symptomatic patients were similar to that in the one asymptomatic patient (L. Zou et al. N. Engl. J. Med. http://doi.org/ggmzsp; 2020).
However, another study found that people with milder COVID-19 symptoms on admission to hospital had much lower concentrations of viral RNA than did those with more severe symptoms (Y. Liu et al. Lancet Infect. Dis. http://doi.org/dqrr; 2020). Wei Zhang at The First Affiliated Hospital of Nanchang University, China, Leo Poon at the University of Hong Kong, and their colleagues say the findings suggest that viral RNA concentrations could predict whether infected people will develop more severe symptoms.
7 April — A comparison finds subtle differences between tests for the COVID-19 virus
Doctors rely on a test called quantitative reverse-transcription polymerase chain reaction (qRT-PCR) to determine whether a person is infected with SARS-CoV-2. A team led by Nathan Grubaugh at Yale School of Public Health in New Haven, Connecticut, compared nine widely used versions of the test and found that all of them reliably detect the virus (C. B. F. Vogels et al. Preprint at medRxiv https://www.medrxiv.org/content/10.1101/2020.03.30.20048108v1; 2020; not peer reviewed before posting).
But the researchers also found that some tests — including one made by the US Centers for Disease Control and Prevention, another developed at Hong Kong University, and a third from Charité–Universitätsmedizin Berlin — performed best when it came to detecting low levels of the virus in samples.
5 April — Bats harbour a pool of coronaviruses related to pandemic culprit
Viruses closely related to SARS-CoV-2, the virus causing the COVID-19 pandemic, have been circulating in horseshoe bats, ready to jump to humans, for decades — and maybe even longer.
David Robertson at the University of Glasgow, UK, and his colleagues analysed the RNA of 68 coronaviruses, including SARS-CoV-2 and the virus that causes severe acute respiratory syndrome, or SARS (M. F. Boni et al. Preprint at bioRxiv https://doi.org/10.1101/2020.03.30.015008; 2020; not peer reviewed before posting). This analysis shows that horseshoe bats (Rhinolophus spp.) host an expanding lineage of viruses that, like SARS-CoV-2, can infect humans. The team estimates that the ancestor of SARS-CoV-2 split 40 to 70 years ago from the closely related bat virus RaTG13. Though the two viruses are highly similar genetically, RaTG13 doesn’t infect humans.
The analysis also suggests that viruses in the lineage are ready to jump to humans directly from bats. But SARS-CoV-2 might have first hopped to another species that humans are more exposed to, rather than spreading straight from bat to human.
3 April — Masks could cut spread of COVID-19 virus
Surgical face masks effectively block the spread of seasonal coronaviruses in respiratory droplets, suggesting that masks could prevent transmission of SARS-CoV-2.
Seasonal coronaviruses are one cause of the common cold. Benjamin Cowling at the University of Hong Kong and his colleagues had ill volunteers who were infected with seasonal coronaviruses sit in an enclosed booth and place their faces in a sampling device, called the Gesundheit-II, that captures airborne particles (N. H. L. Leung et al. Nature Med. https://doi.org/10.1038/s41591-020-0843-2; 2020).
The scientists detected coronavirus RNA in both coarse droplets and finer ‘aerosol’ droplets emitted by volunteers who were not wearing masks. Mask reduced detection of viral RNA in both types of droplet. Larger particles are carried by sneezes and coughs, whereas exhaled breath can spread aerosol droplets, which have a diameter of five micrometres or less.
The authors say that surgical masks reduce transmission of not only seasonal coronaviruses, but also influenza.
Correction: An earlier version of this article said masks reduced detection of viral DNA.
1 April — Antibodies from llamas help to foil the COVID-19 virus
Antibodies from llamas (Lama glama) could help in the fight against several coronaviruses that infect humans.
A team led by Bert Schepens and Xavier Saelens of the VIB life-sciences institute in Ghent, Belgium, and Jason McLellan of the University of Texas at Austin has isolated two llama antibodies that bind the ‘spike’ proteins that coronaviruses use to enter cells (D. Wrapp et al. Preprint at bioRxiv https://doi.org/10.1101/2020.03.26.010165; 2020; not peer reviewed before posting). One antibody neutralized the coronavirus responsible for Middle East respiratory syndrome (MERS); the second mopped up the severe acute respiratory syndrome (SARS) coronavirus.
Fusing the SARS antibody from a llama with an antibody from a human yielded a hybrid that neutralized the virus responsible for COVID-19. The data suggest that such antibodies could be useful in combating coronavirus epidemics
30 March — Debilitated patients rally after dose of survivors’ blood
People seriously ill with COVID-19 experienced striking improvement after receiving infusions of blood from disease survivors, according to two separate research teams.
Both teams extracted antibody-laden plasma — a component of blood — from people who’d recovered from COVID-19.
Xiaoming Yang at the National Engineering Technology Research Center for Combined Vaccines in Wuhan, China, and his colleagues gave the plasma to ten severely ill people. By the sixth day after the treatment, the virus that causes COVID-19 was undetectable in seven of the ten. The recipients experienced no significant side effects (K. Duan et al. Preprint at medRxiv http://doi.org/dqrs; 2020; not peer reviewed before posting).
A group led by Lei Liu at Shenzhen Third People’s Hospital in China gave survivors’ plasma to five “critically ill” people (C. Shen et al. J. Am. Med. Assoc. http://doi.org/dqn7; 2020). Symptoms dwindled in all five; within ten days of receiving the plasma, three recipients no longer needed ventilators.
Other researchers would like to try such transfusions to treat health workers who have been directly exposed.
27 March — Viral proteins point to potential treatments
A list of the human proteins affected by the SARS-CoV-2 virus offers a guide to potential treatments for infected people.
A team led by Nevan Krogan at the University of California, San Francisco, engineered human cells to produce one of 26 proteins made by the coronavirus (D. E. Gordon et al. Preprint at bioRxiv https://doi.org/10.1101/2020.03.22.002386; 2020; not peer reviewed before posting). This allowed the researchers to identify human proteins that physically interact with coronavirus proteins.
Out of 332 interactions between human and viral proteins, the authors identified 67 that existing or candidate drugs could potentially disrupt. The researchers and their collaborators are now testing some of these compounds for antiviral activity — and urge others to do the same.