Peptide synthesis
All peptides were prepared using ChemMatrix Rink amide resin and Fmoc-based automated peptide synthesis on a Prelude X peptide synthesizer (Gyros Protein Technologies) with induction heating and oscillation mixing. All of the solutions were freshly prepared immediately before synthesis as stock solutions in DMF: Fmoc-protected amino acid (0.2 M), HCTU (0.5 M), DIPEA (1.0 M) and piperidine (20% v/v). Peptide elongation was achieved through consecutive cycles of Fmoc deprotection and coupling reactions. Fmoc deprotection was achieved with 20% piperidine in DMF (twice for 2 min, room temperature, 300 rpm shaking) and peptide couplings were performed as double or triple couplings (twice for 5 min, 75 °C, 300 rpm shaking, except for Arg and His, for which twice for 5 min, 50 °C, 300 rpm shaking) consisting of AA/HCTU/DIPEA (ratio, 1:1.25:2.5) in fivefold excess compared to the resin. Extensive DMF washes were performed after each deprotection or coupling reaction.
Peptide cleavage and purification
Dried peptide-containing resin was suspended in 1.5 ml cleavage cocktail (2.5% DODT, 2.5% H2O, 2.5% TIPS in TFA) per 100 mg resin and agitated for 2 h. The peptide-containing solution was collected by filtration, reduced under a stream of nitrogen and precipitated with ice-cold ether. The crude peptide pellet was isolated by centrifugation at 3,600g for 10 min at 4 °C, redissolved in MeCN:H2O (1:1) and lyophilized. Ultraperformance LC (UPLC) and electrospray ionization LC–MS (ESI-LC–MS) analysis were conducted for the crude peptide. Purifications were conducted by preparative RP-HPLC, eluting with a linear gradient (20 ml min−1) and using a binary solvent system consisting of H2O:MeCN:TFA (buffer A, 95:5:0.1; buffer B: 5:95:0.1). The collected fractions were analysed by UPLC and ESI-LC–MS. Fractions with a purity of greater than 95% were pooled and lyophilized. All peptides and peptide drug conjugates were desalted by three consecutive cycles of redissolving the peptide in 0.001 M aqueous HCl followed by lyophilization. All peptides and conjugates used for in vitro and in vivo experiments were of >95% purity.
Synthesis of disulfide linker functionalized (+)-MK-801 and inactive MK-801
2-Mercaptoethanol (Sigma-Aldrich) was treated with 2,2-dipyridyl disulfide (3 equivalents) (Sigma-Aldrich) in dry methanol for 2 h. After completion as monitored by UPLC–MS, the reaction was concentrated in vacuo. Purification by silica gel flash chromatography (EtOAc:CH2Cl2, 2:8) afforded intermediate 2-(pyridine-2-yldisulfaneyl)ethan-1-ol (> 95%). The compound was dissolved in dry CH2Cl2 under an N2 atmosphere and reacted with 4-nitrophenyl chloroformate (1.2 equivalents) for 4 h. The reaction was worked up by extraction, washing with water (×3) and brine. The organic layer was dried over anhydrous MgSO4, filtered and concentrated in vacuo. The crude residue was purified by silica gel flash column chromatography (n-heptane:EtOAc, 2:1) yielding 4-nitrophenyl (2-(pyridin-2-yldisulfaneyl)ethyl) carbonate (89%). The intermediate was finally reacted with the appropriate amine-containing drug (+)-MK-801 (2,2-diphenyl-1-amine; 1.5 equivalent) in dry DMF with addition of dry Et3N (3.0 equivalents) under an N2 atmosphere for 18 h. Purification by preparative HPLC afforded the disulfide linker functionalized (+)-MK-801 (70%) and inactive MK-801 (70%). Conjugation to the corresponding peptides was conducted using a general disulfide conjugation protocol.
Disulfide conjugation protocol
The pure thiol-containing peptide and thiopyridyl-activated linker-functionalized MK-801 were dissolved in DMF (2 ml) and buffer consisting of 6 M guanidine and 1.5 M imidazole in H2O at pH 8 (200 µl) and agitated for at least 2 h. After completion, as monitored by UPLC and ESI-LC–MS, the reaction mixture was diluted with buffer A, filtered and purified directly by preparative RP-HPLC, eluting with a linear gradient (see the ‘Peptide cleavage and purification’ section).
In vitro human plasma stability
Human plasma was preheated at 37 °C for 15 min before being spiked with a final concentration of 0.25 mM of the peptide or conjugate. The samples were collected at timepoints [0, 1, 2, 4, 8, 24 h], [0, 2, 4, 8, 24, 48 h] or [0, 4, 8, 24, 48, 72 h] depending on stability. The samples were processed by pretreatment with 6 M urea for 30 min at 0 °C followed by 20% trichloroacetic acid in acetone (wt./v%) at −20 °C overnight. The samples were centrifuged (13,400 rpm) for 30 min, and the supernatant was collected and filtered (0.2 µm syringe filter) into an LC–MS vial. The samples were analysed by reversed-phase UPLC (RP-UPLC) at 214 nm and LC–MS. The area under the curve was determined and normalized to the first timepoint. Regression lines were computed with GraphPad Prism 9.0 using the one-phase decay equation.
In vitro stability assay with high glutathione concentration
GLP-1–MK-801 was dissolved in dimethyl sulfoxide at a concentration of 0.25 mM and diluted with a solution of 200 mM glutathione in PBS, pH 7, such that the final concentration of GLP-1–MK-801 in the assay was 100 µM. The solution was incubated at 37 °C and samples collected at timepoints [0, 1, 2, 4, 8, 12 h]. The samples were analysed using RP-UPLC at 214 nm and LC–MS. The data were normalized to the first timepoint. Regression lines were computed with GraphPad Prism 9.0 using the one-phase decay equation.
GLP-1 receptor activation
GLP-1 receptor activation was determined using an in vitro bioluminescence resonance energy transfer (BRET)-based assay that measures changes in intracellular cyclic-AMP levels. HEK293 cells were transiently transfected with GLP-1 receptor and cyclic-AMP sensor using YFP-Epac-RLuc (CAMYEL). Cells were cultured at 37 °C in Dulbecco’s modified Eagle’s medium (DMEM) + GlutaMax 1965 (Gibco) supplemented with 10% fetal bovine serum and 1% penicillin–streptomycin in a humidified 10% CO2 incubator.
Cells were seeded in 96-well plates at a density of 35,000 cells per well and transiently transfected using Lipofectamine 2000 according to the manufacturer’s protocol. On the day of the assay, plates were removed from the incubator and each well was washed twice with 100 μl HBSS (Gibco, Life Technologies) and pre-incubated for 30 min at 37 °C with 85 μl HBSS per well. Luciferase substrate coelenterazine (5 μM, Thermo Fisher Scientific, C6780) was added and a baseline was measured after a 5 min incubation. The ligand mixture was added and measurements were recorded every minute for 30 min on a CLARIOstar Plus plate reader (BMG labtech). Dose–response curves were generated at equilibrium (10 min) and EC50 values were calculated from this.
SPR biosensing of human serum albumin binding affinity
Interaction of peptide–drug conjugates with surface immobilized human serum albumin (HSA) (Sigma-Aldrich) was determined using surface plasmon resonance (SPR). The samples were acquired at 25 °C according to literature procedure using a Biacore X100 instrument equipped with a CM5 sensor chip (GE Healthcare Biosciences)48.
The system was equilibrated with 10 mM PBS at a flow rate of 20 µl min−1 to achieve a stable baseline. HSA was immobilized to the surface by pre-activation (1.0 EDC, 1.0 M NHS in PBS, pH 7.4, 7 min) followed by injection of HSA to flow cell 1 (30 µg ml−1 in 10 mM NaOAc buffer, pH 5.0, twice for 7 min). Unreacted sites were capped with ethanolamine (1.0 M ethanolamine in 10 mM PBS, pH 8.1, 7 min). Residual unreacted HSA was removed by three consecutive pulses of 9 s with 25 mM NaOH (3 µl), resulting in a surface of 14,360 RFU.
Interactions between immobilized HSA and the experimental compounds were determined using a running buffer of 10 mM PBS, 3% dimethyl sulfoxide (DMSO), pH 7.4 and a flow rate of 30 µl min−1. Peptides were dissolved in running buffer and the stock concentrations determined using the NanoDrop 2000 spectrophotometer (Thermo Fischer Scientific) at a wavelength of 280 nm. The samples were measured as triplicates by the same researcher, going from low to high concentrations. Compounds were injected for 150 s, and the corresponding dissociation constants were measured for 300 s. The surface was washed between samples with 25 mM NaOH for 4 s followed by equilibration with running buffer. Blanks were acquired after each triplicate measure.
Mouse studies
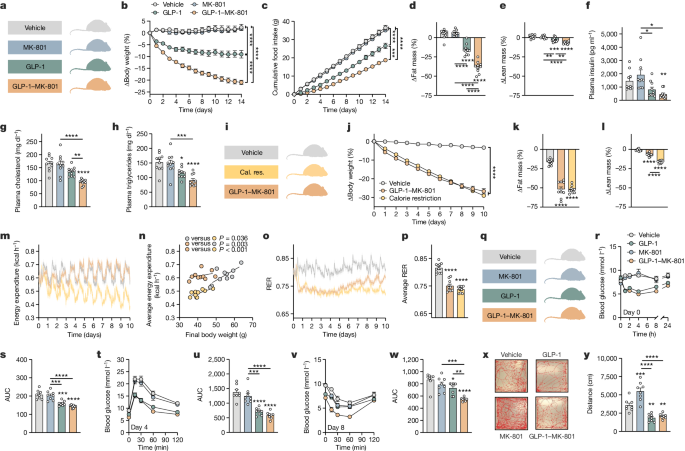
All in vivo experiments were conducted according to international principles of animal care and under the approval of the Danish Ethical Committee for Animal Research and the Danish Animal Experimentation Inspectorate. Experiments were conducted using DIO male C57BL/6J mice (Janvier Labs) kept on a HFHS diet (58 kcal% fat, D12331i, Research Diets) from 8 weeks of age. Mice were maintained on the HFHS diet for a minimum of 16 weeks and had an average body weight of >45 g, before initiation of pharmacological studies. The mice were either single-housed or doubled-housed and maintained on a 12 h–12 h dark–light cycle (06:00–18:00) at 21–23 °C. Mice received once-daily sham injections with isotonic saline from 3 days before study start and were randomized to treatments on the basis of body weight at the day of study start. All compounds were administered as once-daily s.c. injections (between 15:00 and 18:00) with concomitant measurements of body weight and food intake. Vehicle was isotonic saline, which was also used for dissolving the compounds. Compounds were administered at the indicated doses at a volume of 5 µl g−1. For studies with a calorie-restricted and body-weight-loss-matched control group, HFHS diet (58 kcal% fat, D12331i, Research Diets) was weighed and provided as one separate pellet per mouse at the time of injection. The pellets were placed in each side of the cage for double-housed mice and the mice were treated with isotonic saline.
db/db mice (The Jackson Laboratory, 000697) were kept on a chow diet (Brogaarden, Altromin, 1310) and experiments were conducted on 9-week-old male mice. Grouping was based on blood glucose level (using a minimum of 11.1 mM) and body weight. Male Mc4r-KO mice were kept on a HFHS diet for 9 weeks and the mice were 17 weeks old at study start (The Jackson Laboratory, 032518). For electrophysiological studies, male pathogen-free mice at an age of 6–18 weeks were used for all experiments. All of the mice were housed under standard laboratory conditions (12 h–12 h on–off; lights on at 07:00) and a temperature-controlled environment with food and water available ad libitum. All experiments were performed in accordance with the guidelines established by the National Institute of Health Guide for the Care and Use of Laboratory Animals and approved by the University of Texas Institutional Animal Care and Use Committee. To identify POMC neurons and GLP-1 receptor-positive neurons, POMC humanized Renilla green fluorescent protein (hrGFP) (The Jackson Laboratory, 006421) and GLP-1 receptor cre::tdTomato (The Jackson Laboratory, 029283) mice were used. For calcium imaging studies, male Naval Medical Research Institute (NMRI) mice (Taconic Biosciences, BomTac:NMRI) were used (aged 18–28 days). Mice were bred and housed in the animal facility at the Department of Drug Design and Pharmacology, University of Copenhagen and studies were conducted under the approval of the Danish Ethical Committee for Animal Research and the Danish Animal Experimentation Inspectorate. The mice were housed in ventilated cages in a humidity- and temperature-controlled room (temperature, 22 ± 2 °C; humidity, 36–58%) with a 12 h–12 h light–dark cycle (lights on at 07:00) with ad libitum access to chow diet and tap water.
Rat studies
Double-housed male Sprague-Dawley rats (Janvier Labs, RjHan:SD) were kept on a HFHS diet for 4 weeks from 8–10 weeks of age and had an average body weight of >500 g before initiation of pharmacological studies. The rats were housed in ventilated cages in a humidity- and temperature-controlled room (temperature, 22 ± 2 °C; humidity, 36–58%) under a 12 h–12 h light–dark cycle (lights on at 07:00) with ad libitum access to chow diet and tap water. The rats were administered once-daily s.c. injections (between 15:00 and 17:00) of 100 nmol kg−1 MK-801, 10 nmol kg−1 semaglutide, 100 nmol kg−1 GLP-1–MK-801 or vehicle, with concomitant measurements of body weight and food intake. Vehicle was isotonic saline and compounds were administered at a volume of 1 µl g−1. For the kaolin-intake study in Sprague-Dawley rats, 8-week-old double-housed male rats were used.
For CTA and kaolin-intake studies, male Wistar rats (Janvier Labs, WistarRjHan:WI) were used. The rats were 8 weeks old at the initiation of the studies with a body weight of 250–325 g. The rats were group-housed (3–4 rats per cage) in ventilated cages in a humidity- and temperature-controlled room (temperature, 21 ± 2 °C; humidity, 50 ± 10%) under a 12 h–12 h light–dark cycle (light, 01:00 to 13:00) with ad libitum access to chow diet (Brogaarden, Altromin, 1324) and tap water. The rats were dosed by s.c. injections of 100 nmol kg−1 MK-801, 100 nmol kg−1 GLP-1, 10 nmol kg−1 semaglutide, 100 nmol kg−1 GLP-1–MK-801 or vehicle immediately before onset of the dark cycle at a volume of 2 ml kg−1.
Tissue collection and processing
Mice or rats were euthanized by decapitation for collection of trunk blood and tissues. Blood was collected in EDTA-coated microvette tubes, chilled on ice and centrifuged at 3,000g and 4 °C for 10 min. The plasma was aliquoted and stored at −80 °C until analysis. Tissues were collected by dissection, frozen on dry-ice and stored at −80 °C until further processing.
Body composition measurements
Body composition measurements were performed using quantitative nuclear magnetic resonance imaging (EchoMRI).
Glucose tolerance, compound tolerance and insulin tolerance tests
Mice were fasted for 5 h before being challenged with an intraperitoneal injection of 1.75 g kg−1 of glucose dissolved in isotonic saline. Tail vein blood glucose concentrations were measured using a handheld glucometer (Contour XT, Bayers) at 0, 15, 30, 60 and 120 min after injection (for the Mc4r KO study a 90 min timepoint was also included). Compound tolerance was assessed by s.c. injection of the experimental compound followed by measurements of tail vein blood glucose concentrations using a handheld glucometer (Contour XT, Bayers) at various timepoints over the following 24 hours. For studies with measurements of plasma insulin and glucagon concentrations, plasma was collected by tail vein bleeding at timepoints 0, 60, 120 and 240 min. For insulin tolerance testing, the mice were fasted for 5 h before being challenged with an intraperitoneal injection of 0.75 U kg−1 of human insulin (Actrapid). Tail vein blood glucose concentrations were measured using a handheld glucometer (Contour XT, Bayers) at 0, 15, 30, 60 and 120 min after injection. For glucose-stimulated insulin secretion testing, the mice were fasted for 4 h before being challenged with intraperitoneal injections of 1.75 g kg−1 of glucose dissolved in isotonic saline. Tail vein blood glucose concentrations were measured using a handheld glucometer (Contour XT, Bayers) at 0, 15, 30, 60 and 120 min after injection. Plasma was collected at timepoints 0, 15 and 60 min by tail vein blood sampling and insulin concentrations were measured by enzyme-linked immunosorbent assay (ELISA; Crystal Chem Ultra Sensitive Mouse Insulin Eisa Kit, 90080).
Rectal body temperature
Conscious mice were restrained and a high-precision thermometer (BIO-TK8851, Bioseblab) was carefully inserted half-way into the rectum. Temperature measurements of each mouse were performed by the same researcher on day 7 at timepoints 0, 20, 45 and 90 min in response to s.c. injection with 200 nmol kg−1 MK-801, 600 nmol kg−1 MK-801 or vehicle, and for measurement of rectal temperature on day 14 of mice receiving 14 days of once-daily s.c. dosing with 100 nmol kg−1 MK-801, 100 nmol kg−1 GLP-1, 100 nmol kg−1 GLP-1–MK-801 or vehicle.
Plasma parameters
Plasma was sampled from a non-fasted state 2 h after the final compound administration. Plasma insulin levels were quantified using the Crystal Chem Ultra Sensitive Mouse Insulin ELISA kit (Crystal Chem Ultra Sensitive Mouse Insulin Eisa Kit, 90080). Plasma glucagon levels were quantified using the Mercodia Glucagon ELISA kit (10-1281-01, Mercodia). Plasma total cholesterol (Thermo Fisher Scientific, Infinity Total Cholesterol Reagent, TR13421), triglycerides (total glycerol and triglycerides) (Thermo Fisher Scientific, Infinity Total Triglycerides Reagent, TT22421), non-esterified fatty acids (NEFA) (Invitrogen, non-esterified free fatty acids (NEFA/FFA) Colorimetric Assay Kit, Thermo Fisher Scientific, EEA017), AST (Thermo Fisher Scientific, EEA003) and ALT (Thermo Fisher Scientific, EEA001) were quantified using enzymatic kits according to the manufacturer’s protocols.
In vivo pharmacokinetic measurements
The pharmacokinetic assessment was conducted using a total of eight male DIO C57BL/6J mice (n = 4 per subgroup). Mice were administered single s.c. injections with a dose of 100 nmol kg−1 of the experimental compounds. Each subgroup was bled at the following timepoints: subgroup A at 15 min, 45 min, 2 h and 8 h; subgroup B at 30 min, 1 h, 4 h and 24 h.
Mouse plasma containing test substance was crashed in 96-well non-binding plates using liquid–liquid extraction with ethanol containing internal standard. The samples were then centrifuged, and the supernatants were transferred to new wells and diluted with water. The prepared mouse plasma was analysed for the test substance using LC–MS. The system consisted of a TSQ Quantis Triple Quad mass spectrometer (Thermo Fisher Scientific) equipped with a Vanquish Horizon UPLC (Thermo Fisher Scientific). RP-UPLC separation was performed on the Acquity UPLC system (Waters, column: BEH C18 1.7 µm, 2.1 × 50 mm). Mobile phase A was composed of 0.1% formic acid in water and mobile phase B was composed of 0.1% formic acid in acetonitrile. The UPLC flow rate was set to 0.3 ml min−1 at 60 °C using a gradient elution from 10 to 65% B over the course of 4.0 min. The gradient was then ramped from 65% B to 99% B for 0.1 min and held at 99% for 0.9 min. The mass spectrometer was operated in positive-ionization SRM mode.
Intracerebroventricular administration study
The intracerebroventricular study was performed in HFHS-fed C57BL/6J mice that were single-housed in open cages. Mice were pretreated with lidocaine (Accord Healthcare) at the site of incision, anaesthetized with isoflurane and fixed in a stereotaxic instrument. The skin of the head was incised, a hole was drilled into the skull and a guide cannula was subsequently implanted into the lateral ventricle (26GA; PlasticOne; C2315GS-4/SPC) using stereotaxic coordinates (−0.3 mm posterior to bregma; ±1.0 mm lateral to bregma) (David Kopf Instruments). The guide cannula was held in place using UV-cured cement (G-bond and G-aenial Universal Flo, GC), and a dummy cannula (PlasticsOne, C315DCS-4/Spc, 2.5 mm) was inserted into the guide cannula to keep the cannulation closed until and in between compound infusions. Post-operatively, mice were administered s.c. injections of carprofen (5 mg kg−1, Pfizer) for 3 days and allowed to recover for a minimum of 7 days with daily monitoring of food intake and body weight.
After full recovery, correct cannulation was tested by observing water drinking responsiveness after infusion of 1 µl human angiotensin II (Sigma-Aldrich, A9525) at a concentration of 24 µM in artificial cerebrospinal fluid (distilled water with 125 mM NaCl, 2.5 mM KCl, 2.6 mM NaHCO3, 1.25 mM NaH2PO4·2H2O, 25 mM d-glucose monohydrate, 1 mM MgCl2, 2 mM CaCl2). Mice that did not drink within 15 min of the infusion were excluded from the study. In the afternoon on the experimental day, semaglutide (Novo Nordisk) and GLP-1–MK-801 were dissolved in isotonic saline at a concentration of 0.11 nmol µl−1. Before infusion, mice were relocated to new cages with fresh bedding to ensure that no remnant food pellets remained in the cage. Mice were placed onto a cage hopper and infused with semaglutide, GLP-1–MK-801 or vehicle (isotonic saline) over 60 s at a total infusion volume of 2 µl through a Hamilton syringe mounted onto an automated syringe pump (Harvard Apparatus) and a 33-gauge internal cannula (PlasticsOne, C315IS-4/SPC, 2.5 mm). The injector was kept in the guide cannula for 30–60 s after infusion stop to ensure complete infusions and to avoid backflow. Body weight and food intake were subsequently monitored daily in the afternoon. At day 6 after the first infusion, the semaglutide-treated and GLP-1–MK-801-treated mice were crossed-over in terms of treatment and administered with another single infusion of either GLP-1–MK-801 (0.22 nmol, 2 µl) and semaglutide (0.22 nmol, 2 µl), respectively, while vehicle-treated mice received vehicle treatment once again. Again, body weight and food intake were monitored once daily in the afternoon.
Metabolic phenotyping and indirect calorimetry
Single-housed male DIO C57BL/6J mice were acclimatized to metabolic cages (16-channel Promethion, Sable Systems International) for 1 week before the start of the study. Oxygen consumption (VO2), carbon dioxide production (VCO2), RER, energy expenditure (kcal h−1) and locomotor activity (cm s−1) were recorded and collected in 15 min bins. Water and HFHS food were available ad libitum throughout the study period. New food was provided every second day. For study 1 (weight loss study of GLP-1–MK-801 relative to monotherapies), mice were randomly divided into four experimental groups (n = 8 mice per group) with similar mean body weights and assigned to receive once-daily s.c. injections of 100 nmol kg−1 MK-801, 100 nmol kg−1 GLP-1, 100 nmol kg−1 GLP-1–MK-801 or vehicle for 14 days. For study 2, mice were randomly divided into three experimental groups (n = 10 mice per group) such that each group had a similar mean body weight and was assigned to receive once-daily s.c. injections with 100 nmol kg−1 GLP-1–MK-801 or vehicle, or calorie restriction to match the weight loss trajectory of the GLP-1–MK-801 group for 10 days. In both studies, body weights and food intake were measured manually each day at the time of injection and dosing was performed at a volume of 5 µl g−1. Mice assigned to the different experimental groups were randomly distributed across two systems. Raw data for each individual mouse were analysed using the online tool CalR (CalR, v.1.3; www.calrapp.org) and visualized using GraphPad Prism. ANCOVA analyses for statistical comparison of regression lines in body weight versus average energy expenditure plots were computed using CalR.
Open-field test
Locomotor activity was evaluated using an open-field test. Mice were acclimatized in the procedure room for 7 days before the experiments. The experiments were conducted by placing the mice in one of four 50 × 50 × 50 cm arenas immediately after compound administration, allowing their locomotion to be monitored by a ceiling-mounted Logitech C920 Pro camera (1,080 × 1,080 px, 30 fps, Logitech software). DIO mice were divided into four groups such that each group had the same average mean body weight (n = 8 mice), and their movements were recorded for a period of 20 min. Each run was conducted with one mouse from each treatment group and with run-to-run alternation so that treatments were equally distributed across all four arenas. Movement traces and quantification of locomotor activity (velocity and distance travelled) were obtained using Noldus EthoVision XT software (Noldus).
CTA assay
A week before the study start (day −7), rats were single-housed with ad libitum access to chow diet and one bottle of water (food and water were placed in the cage lid so that there was room for two water bottles). The position of the water bottle was alternated daily between sides to avoid the development of a side preference. The animals were weighed and handled daily from day −3. On day −3, the rats were exposed to a bottle containing 0.1% saccharin-flavoured water with high palatability followed by s.c. administration of the experimental compounds (MK-801, GLP-1, GLP-1–MK-801 (all at doses of 100 nmol kg−1), semaglutide (10 nmol kg−1) or isotonic saline as vehicle). Then, 3 days after the first dosing, the rats were exposed to a two-bottle taste preference test, that is, a voluntary choice between tap-water or the 0.1% saccharin solution. Saccharin and water intake was monitored for 24 h followed by preference determination. These experiments were conducted at Gubra.
Kaolin intake
For the Wistar pica study, rats were group-housed (3–4 rats per cage) and allowed to acclimatize to the HM-2 system (MBRose) for 7 days before the study start. At the initiation of the experiment, the rats were randomized such that each group had the same average body weight and was then assigned to receive once-daily s.c. injections of 100 nmol kg−1 MK-801, 100 nmol kg−1 GLP-1, 100 nmol kg−1 GLP-1–MK-801 or vehicle (isotonic saline). Food intake data were collected on a continuous basis from 24 h before dosing (allowing for replenishment of food as well as dosing time) and throughout the study. After the study start, food pellets were placed in one food channel of the HM-2 system and kaolin pellets in the other channel. The position of the food and kaolin was alternated every day to correct for side preference. These experiments were conducted at Gubra. For the Sprague Dawley study, rats were single housed and had ad libitum access to a chow diet, kaolin pellets (K50001, Research Diets) and tap water. The rats were habituated to kaolin for 5 days before pharmacological testing. At the initiation of the experiment, the rats were randomized such that each group had the same average body weight and was then assigned to receive once-daily s.c. injections of 100 nmol kg−1 MK-801, 100 nmol kg−1 GLP-1, 100 nmol kg−1 GLP-1–MK-801 or vehicle (isotonic saline) for 3 days. Body weight, chow intake and kaolin intake were measured daily, and the cage was carefully assessed for any remnant leftover food and kaolin.
Voluntary running
Experiments were conducted using 8-week-old male C57BL/6J mice (Janvier Labs) kept on a chow diet. Mice were single-housed in cages equipped with a running wheel (23 cm in diameter, Techniplast). The amount of bedding material was reduced to avoid blockade of the running wheels. Running distance was monitored using a Sigma Pure 1 Topline 2016 computer (Sigma Sports) and, after 1 week of habituation, daily running distance was monitored for 3 days. The effect of experimental compounds on voluntary wheel running was monitored by randomizing mice to receive once-daily s.c. injections of 100 nmol kg−1 GLP-1–MK-801, 10 nmol kg−1 semaglutide or vehicle (isotonic saline) based on baseline running wheel distance measurements, such that each group had similar average running wheel distances at the day of study initiation. Running distance, food intake and body weight were measured daily before dosing of experimental compounds.
Heart rate and blood pressure assessment
Blood pressure and electrocardiogram (ECG) were recorded in anaesthetized, lean mice. Chow-fed male C57BL/6J mice at 18 weeks of age were randomly assigned to receive once-daily s.c. injections of 100 nmol kg−1 MK-801 (n = 10), 100 nmol kg−1 GLP-1 (n = 10), 100 nmol kg−1 GLP-1–MK-801 (n = 10) or vehicle (isotonic saline, n = 10) for 14 days. Body weight and food intake were measured manually each day at the time of injections. The morning after the final injections, mice were anaesthetized using 2% isoflurane in a mix of O2 and N2 (30:70). The mice received an intraperitoneal administration of isotonic saline (0.5 ml) to compensate for potential fluid loss. Body temperature was monitored and kept at 36–37 °C throughout the procedure.
For heart rate measurements, a surface ECG was recorded using custom-made needle electrodes placed on each limb. For blood pressure measurements, a 0.8F pressure catheter (SPR-1000, Millar, 8410001) connected to a bioamplifier unit was inserted in the right carotid artery. The catheter tip was placed at the level of the aortic arch. Evaluation of blood pressure pulse profile was used to confirm correct positioning of the catheter tip. A PowerLab unit (PowerLab 16/35, AD Instruments) was used to record ECG and blood pressure at a sampling rate of 4 kHz. The ECG was recorded for 5 min after the mouse was anaesthetized and then again for 10 min once the blood pressure catheter was placed.
Electrophysiology
Brain slices were prepared from adult Glp1r-cre::tdTomato or Pomc-hrGFP male mice (aged 6–18 weeks) as previously described49,50,51. In brief, male mice were deeply anaesthetized with an intraperitoneal injection of 7% chloral hydrate and transcardially perfused with a modified ice-cold artificial cerebrospinal fluid (aCSF) (described below). The mice were then decapitated and the entire brain was removed and immediately submerged in ice-cold, carbogen-saturated (95% O2 and 5% CO2) aCSF (126 mM NaCl, 2.8 mM KCl, 1.2 mM MgCl2, 2.5 mM CaCl2, 1.25 mM NaH2PO4, 26 mM NaHCO3 and 5 mM glucose). Coronal sections (250 mm) were cut using the Leica VT1000S Vibratome and then incubated in oxygenated aCSF (32–34 °C) for at least 1 h before recordings. The slices were bathed in oxygenated aCSF (32–34 °C) at a flow rate of ~2 ml min−1. All electrophysiology recordings were performed at ambient temperature.
The pipette solution for whole-cell recordings was modified to include an intracellular dye (Alexa Fluor 350 hydrazide dye) and contained: 120 mM K-gluconate, 10 mM KCl, 10 mM HEPES, 5 mM EGTA, 1 mM CaCl2, 1 mM MgCl2 and 2 mM MgATP, and 0.03 mM Alexa Fluor 350 hydrazide dye (pH 7.3). Epifluorescence was briefly used to target fluorescent cells, at which time the light source was switched to infrared differential interference contrast imaging to obtain the whole-cell recordings (Zeiss Axioskop FS2 Plus equipped with a fixed stage and a QuantEM:512SC electron-multiplying charge-coupled device camera). Electrophysiological signals were recorded using the Axopatch 700B amplifier (Molecular Devices), low-pass filtered at 2–5 kHz, and analysed offline on a PC with pCLAMP programs (Molecular Devices). To measure NMDA-induced inward current from GLP-1-receptor-positive neurons, we used magnesium-free aCSF (126 mM NaCl, 2.8 mM KCl, 2.5 mM CaCl2, 1.25 mM NaH2PO4, 26 mM NaHCO3 and 5 mM glucose) containing 10 μM CNQX and 100 μM picrotoxin. Membrane potentials and firing rates were measured from POMC neurons in brain slices. Recording electrodes had resistances of 2.5–5 MΩ when filled with the K-gluconate internal solution. The frequency and peak amplitude of excitatory neurons were analysed using the Easy electrophysiology program (Easy Electrophysiology).
Drug working concentrations and stock preparation were as follows: GLP-1–MK-801 and MK-801 (both 50 μM, dissolved in aCSF or magnesium-free aCSF), NMDA (100 μM, dissolved in magnesium free aCSF), CNQX (10 μM, dissolved in DMSO, Alomone Labs), picrotoxin (100 μM, dissolved in DMSO). The final concentration of DMSO applied to the slices was <0.05%.
A change in membrane potential was required to be at least 2 mV in amplitude in response to drug application. Membrane potential values were not compensated to account for junction potential (−8 mV). Effects of GLP-1–MK-801 on frequency (over 0.5 Hz) and synaptic activity before and during acute GLP-1–MK-801 bath application were analysed within a recording using the Kolmogorov–Smirnov test (a nonparametric, distribution-free goodness-of-fit test for probability distributions).
Calcium imaging
Brain slices were prepared from adult NMRI male mice (aged 18–28 days). After deep anaesthesia with isoflurane (Attane Vet, 1,000 mg g−1, ScanVet, Piramal Critical Care) mice were decapitated and the brain was removed and submerged in ice-cold aCSF (124 mM NaCl, 5 mM KCl, 1.2 mM Na2HPO4·2H2O, 2.7 mM CaCl2·2H2O, 1.2 mM MgSO4 (anhydrous)), 10 mM dextrose, 26 mM NaHCO3 was adjusted to pH 7.4 and an osmolarity of 298–302 mOsm kg−1 after saturation with carbogen (95% O2/5% CO2). A vibratome (Leica VT1200S, Leica Biosystems) was used to obtain 250 μm thin acute brain slices containing Arc, identified by well-known landmarks. After incubation for 15 min in a 32 °C water bath and 1 h at ambient temperature, the slices were loaded with Fura-2-AM (4 mM; Hello Bio) under carbogen exposure in a 32 °C water bath for 10 min + 1 min for each PND. The slices were rinsed and placed into a chamber embedded in the stage of an Olympus BX51WI microscope (Olympus) coupled to a 12-bit CCD fluorescent camera (SensiCam, PCO imaging). A monochromator (Polychrome V, TILL Photonics, FEI) combined with a xenon light bulb provided fluorescent illumination. Protocols for fluorescence exposure of slices were controlled by software (Live Acquisition, TillVision), and analyses were conducted using Offline Analysis (TillVision).
Regions of interest were drawn around Fura-2-AM-loaded cells under ×40 magnification as well as around one region of the field devoid of cells, which was to be used as the background. The entire field of view was exposed to excitation wavelengths of 340 nm (exposure time, 50 ms) and 380 nm (exposure time, 40 ms). Each frame pair (340 nm:380 nm was collected at an interval of 4 s for 10 min of recording in total. GLP-1 or GLP-1–MK-801 (1 µM in aCSF) was bath-applied for 25 min, with recording only being conducted during the first 10 min, at which point a maximum and stable change in fluorescence was achieved. Fifteen additional minutes of application of GLP-1 or GLP-1–MK-801 were conducted without recording to minimize exposure to fluorescent light to reduce bleaching of the fluorescent indicator. Then, a 10 min recording with excitation was started, and after establishment of a baseline consisting of 10 frame pairs, NMDA (50 µM, Tocris) + GLP-1 or GLP-1–MK-801 was bath-applied.
The fluorescence intensity within each region of interest was binned at 2 × 2 pixels and averaged. A ratio of fluorescence intensities measured at 340 nm and 380 nm was calculated minus the background fluorescence. The peak amplitude of a change in fluorescence induced by GLP-1, GLP-1–MK-801 or NMDA was calculated by taking an average of ten datapoints from the baseline (F0) and subtracting this from an average of ten data points from the peak fluorescence (ΔF: average amplitudepeak − average amplitudebaseline), which was normalized by dividing by F0. Graphic plots were converted to a percentage defined as %Δ F/F0.
RNA-seq analysis
mRNA-seq was performed by the Single-Cell Omics platform at the Novo Nordisk Foundation Center for Basic Metabolic Research. Libraries were prepared using the Universal Plus mRNA-seq protocol (Tecan) according to the manufacturer’s protocol. Libraries were quantified with NuQuant, quality checked using a TapeStation instrument (Agilent Technologies) and subjected to 52 bp paired-end sequencing on the NovaSeq 6000 system (Illumina). For differential expression testing, the R package DESeq2 (v.1.30.1) was used to identify differentially expressed genes. P values were adjusted for multiple testing using the Benjamini–Hochberg post hoc method. For functional enrichment analysis, the R package gprofiler2 (v.0.2.0) was used to identify enriched functional terms (GO:MF, GO:BP, GO:CC, KEGG pathways and REACTOME pathways). The gene-set enrichment analysis was performed with the parameters ‘exclude_iea’ set to true and ‘correction method’ set to Benjamini-Hochberg. SynGO enrichment analyses were conducted using the online tool https://syngoportal.org/ with the background set to brain expressed and using differentially expressed genes (P < 0.05). The following transcripts are not depicted in Fig. 3e: gh, scarna13 and CT010467.1.
MS-based proteomics
Hypothalamic tissue was powdered and lysed (lysis buffer 50 mM Tris, 4% SDS buffer, pH 8.5) using BeatBox homogenizer (PreOmics). Protein lysates were boiled at 95 °C for 10 min on a thermomixer (Thermo Fisher Scientific) and sonicated on the Bioruptor (Diagenode) system. Proteins were digested into peptides using a high-throughput automated version of the protein aggregation capture workflow52 on the KingFisher Flex Purification System (Thermo Fisher Scientific). Proteins were on-bead digested overnight in a solution containing LysC and trypsin at 37 °C. The resulting tryptic peptides were desalted using in-house-crafted SDB-RPS StageTips and 200 ng of peptides were loaded in Evotips (Evosep) according to the manufacturer’s instructions.
Desalted peptides were separated on the Pepsep (15 cm, 150 μM inner diameter) column packed with C18 beads (1.9 μm; Bruker) on the Evosep ONE HPLC system using the ‘30 samples per day’ method, then injected through a CaptiveSpray ion source and 20 μm emitter into a timsTOF Pro 2 mass spectrometer (Bruker) operated in diaPASEF mode. The resulting MS raw files were processed with the DIA-NN software v.1.876 in a library-free manner, using a Mus Musculus FASTA file from UniProt (December 2023). Proteotypic peptides were used for protein group quantification, under double-pass mode neural network configuration. ‘Robust LC (high accuracy)’ was chosen as the quantification strategy and the match between runs options was enabled. The rest of the parameters were set as the default, which included precursor FDR set to 1% and peptide length of 7–30 amino acids. For differential expression analysis, the R package limma (v.3.54.2) was used to identify differentially expressed proteins. P values were adjusted for multiple testing using the Benjamini–Hochberg post hoc method. For functional enrichment analysis, enriched gene sets were determined by applying the same workflow used for RNA, using the R package gprofiler2 (v.0.2.0). SynGO enrichment analyses were conducted using the online tool https://syngoportal.org/ with the background set to brain expressed and using differentially expressed proteins (P < 0.05).
cFOS whole-brain imaging
Lean male C57BL/6J mice (aged 8 weeks) maintained on a chow diet (Brogaarden, Altrumin, 1310) were randomized 4 days before the study start and treated with once-daily s.c. mock dosing with isotonic saline. Study 1 was conducted during the light phase. All compounds were prepared as solutions in isotonic saline and dosed as s.c. injections of 10 nmol kg−1 semaglutide (n = 8 mice), 100 nmol kg−1 GLP-1–MK-801 (n = 8 mice) or vehicle (isotonic saline, n = 8 mice) at a volume of 5 µl g−1. Study 2 was conducted during the light phase. All of the compounds were prepared as solutions in isotonic saline and dosed as s.c. injections of 100 nmol kg−1 MK-801 (n = 8 mice), 100 nmol kg−1 GLP-1 (n = 8 mice), 100 nmol kg−1 GLP-1–MK-801 (n = 8 mice) or vehicle (isotonic saline, n = 8 mice) at a volume of 5 µl g−1. One outlier was removed from the vehicle, MK-801 and GLP-1–MK-801 groups due to deviation related to tissue processing. Tissue processing and quantification of cFOS was conducted as previously described37. These experiments were conducted at Gubra.
Brain abbreviations are as follows: NAc, nucleus accumbens; PVH, paraventricular hypothalamic nucleus; DMH, dorsomedial nucleus of the hypothalamus; ARC, arcuate nucleus of the hypothalamus; LHA, lateral hypothalamic area; CEA, central amygdala nucleus; SNc, substantia nigra, compact part; VTA, ventral tegmental area; PB, parabrachial nucleus; NTS, nucleus of the solitary tract; DMX, dorsal motor nucleus of the vagus nerve; AP, area postrema; IMD, intermediodorsal nucleus of thalamus; PG, pontine gray; DG, dentate gyrus; MS, medial septal nucleus; LS, lateral septal nucleus; SFO, subfornical organ; PH, posterior hypothalamic nucleus; SLD, sublaterodorsal nucleus; TRN, tegmental reticular nucleus; PSTN, parasubthalamic nucleus; PS, parastrial nucleus; B, Barrington’s nucleus; PVT, paraventricular nucleus of the thalamus; RR, midbrain reticular nucleus, retrorubral area; MD, mediodoral nucleus of thalamus; SUT, supratrigmental nucleus; IRN, intermediate reticular nucleus; LC, locus ceruleus; MDRNd, medullary reticular nucleus, dorsal part; GU, gustatoty areas.
Quantification and statistical analysis
Statistical analyses were performed using GraphPad Prism 10.1.1 (GraphPad) and figures were generated using either GraphPad Prism or CorelDraw X8 (Corel). For comparison of multiple groups, one-way ANOVA or two-way repeated measures ANOVA were used. Two-way ANOVA main effects are reported and Bonferroni post hoc multiple-comparison analyses applied when relevant for interpretation. Regression plot ANCOVA analyses of indirect calorimetry data were computed using the online tool calR (www.calR.org). For comparison of two groups, unpaired two-tailed Students t-tests were used. Data were evaluated for distribution patterns using tests including Shapiro-Wilk and Kolmogorov–Smirnov tests and by visual inspection of the distribution residuals. Data from designated brain regions from the cFOS 3D brain imaging study were analysed using one-way ANOVA with Dunnet’s post hoc multiple-comparison test relative to the vehicle, using a negative binomial generalized linear model to control for Gaussian distribution. However, the top 20 most statistically significantly regulated brain regions in response to treatment were analysed as previously described37. No statistical methods were applied to predetermine the sample size for in vivo pharmacology experiments. Data represent mean ± s.e.m.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.