In the nervous system, synapses are where the action is. There, across a narrow gap between adjacent cells, neurons talk to one another through the dynamic exchange of chemical and electrical signals. Molecules known as neurotransmitters and neuromodulators induce or inhibit action potentials — spikes in voltage across neuronal cell membranes that trigger the release of other molecules. This cross-talk ultimately enables the production of emotions, thoughts, behaviours — everything that makes the brain what it is.
A new way to capture the brain’s electrical symphony
To decode these conversations, researchers have relied on various tools. These include electrophysiology, in which electrodes are inserted into the brain or individual cells (in the case of patch-clamp recording) to measure the voltage changes linked to action potentials; microdialysis, in which some of the fluid surrounding neurons is extracted and analysed; and fast-scan cyclic voltammetry, which uses implanted electrodes to detect certain signalling molecules.
But these methods have limitations. For instance, electrophysiology can precisely measure action potentials, but scientists can’t pinpoint the exact neurotransmitters or neuromodulators (collectively called neurochemicals) that drive them. Microdialysis can identify specific molecules, but it lacks the spatial and temporal resolution to pinpoint exactly when and where these neurochemicals are released, and voltammetry often struggles to distinguish molecules that are similar to each other.
The development of genetically encoded sensors over the past two decades has offered a way for neuroscientists to circumvent these issues. Such sensors were initially developed to identify action potentials in cells by tracking changes in calcium ions, but in recent years, researchers have expanded the toolbox to detect key neurochemicals.
The next generation of these sensors now enables scientists to ask questions such as: how much of a specific neurochemical is released in response to the firing of an action potential? How many action potentials are required to release a given neurochemical? And how long does that molecule stick around? “All these kinds of questions, for the vast majority of molecules — we’re talking about dopamine, serotonin, acetylcholine and many others — we know virtually nothing about,” says Nicolas Tritsch, a neuroscientist at McGill University in Montreal, Canada. “This new class of genetically encoded sensors has really opened up this world.”
Lighting up cells
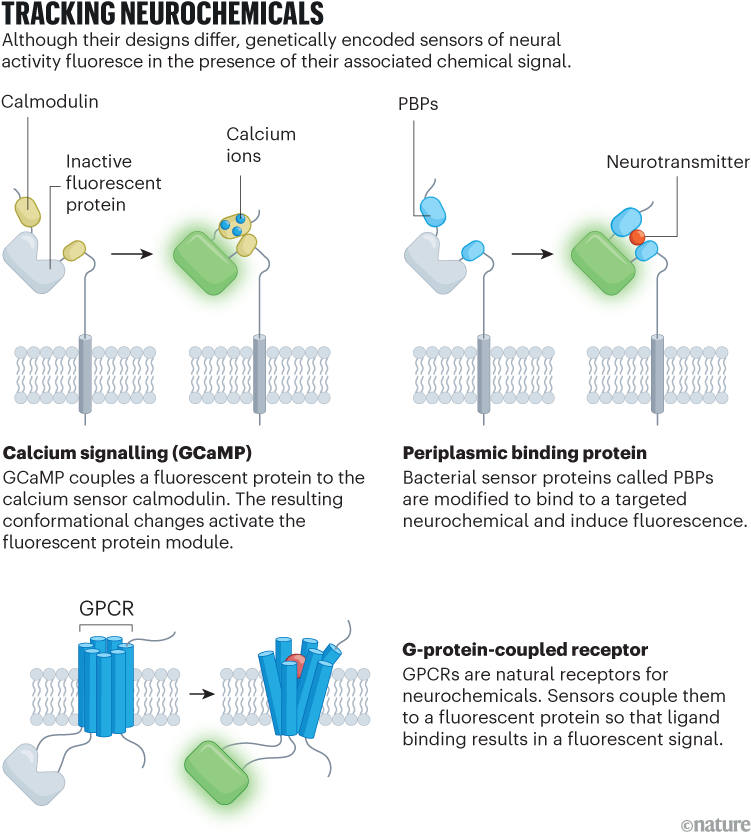
The revolution in genetically encoded neurochemical sensing began with calcium. Action potentials activate specialized channel proteins on the neuron’s cell membrane and allow calcium to enter, changing the calcium concentration. By fusing the calcium-binding protein calmodulin with a fluorescent protein and genetically targeting the hybrid molecule to specific populations of cells, researchers have developed sensors that light up in response to calcium fluctuations — a proxy for neuronal activity.
One popular variety of genetically encoded calcium indicators, known as GCaMPs, has been around since 2001. Researchers have since optimized the sensors’ speed and sensitivity — and they are now mainstays of neuroscience research. “They’re so ubiquitous that most papers that use GCaMPs stopped citing the relevant papers,” says Loren Looger, a neuroscientist at the University of California, San Diego (UCSD), whose team has been developing these sensors.
Genetically encoded sensors offer several advantages (see ‘Tracking neurochemicals’). Researchers can express them at specific times in particular cells, then pair them with techniques such as optogenetics to cause them to fire in response to light. But they also require genetic manipulation and might change the biology of cells in unexpected ways.
Sources: GCaMP: Y. Yang et al. Ann. Rev. Anal. Chem. 17, 367–392 (2024); PBP & GPCR: Z. Wu et al. Nature Rev. Neurosci. 23, 257–274 (2022)
Still, the success of these sensors motivated many tool developers, including Looger, to dream up similar strategies for detecting other neurochemicals. Among them are neurotransmitters such as glutamate, γ-aminobutyric acid (GABA) and acetylcholine — which activate or inhibit nerve cells — and neuromodulators such as dopamine, serotonin and endocannabinoids, which trigger molecular pathways in the brain1.
In 2018, two papers2,3 published just weeks apart by teams in the United States and China independently described GCaMP-like sensors for dopamine, a neuromodulator involved in reward-based decision-making, learning and many other processes.
Dubbed dLight1 and GRAB-DA, respectively, both teams’ sensors were built on the dopamine receptor, one of a family of proteins known as G-protein-coupled receptors (GPCRs). The teams, led by neuroscientist Lin Tian, then at the University of California, Davis, and by Yulong Li, a neuroscientist at Peking University in Beijing, modified the receptor to fluoresce in the presence of dopamine and inserted that gene into a virus. Researchers could then inject the sensors into a living animal and observe the activity of specific molecules in infected cells in the brain using fibre photometry, a technique that measures changes in fluorescence using an optical fibre implanted in the brain.

How to keep the lights on: the mission to make more photostable fluorophores
These sensors quickly became popular additions to the neuroscience toolkit, and the papers have been cited more than 800 times apiece. In part, says Mark Walton, a neuroscientist at the University of Oxford, UK, their popularity is due to their ease of use. Conventional methods can be difficult to master — voltammetry, in particular, “is a bit of a black art”, he says. But with genetically encoded sensors, “we’ve never looked back”. Among other things, Walton and his team have used them to investigate dopamine fluctuations in almost 200,000 tests in mice, revealing that, contrary to previous results from conventional approaches, reward-based decision-making can happen independently of dopamine4.
Another benefit of GPCR-based sensors is their generalizability, with a design that has now been extended to receptors for acetylcholine, serotonin and endocannabinoids. “Those initial dopamine sensors and their subsequently optimized variants have been transformative,” says Matthew Banghart, a neuroscientist at UCSD. “It’s so easy to implement, and the sky’s the limit in terms of being able to apply this approach to any GPCR of interest.”
Enabling discoveries
These sensors have begun to fundamentally shift our understanding of how neurotransmitters and neuromodulators work. Take dopamine, for instance. Although scientists have long known what it does, how exactly it transmits information in the brain has remained a mystery.
As a postdoctoral researcher at Brown University in Providence, Rhode Island, in the late 2010s, Arif Hamid wanted to understand how dopamine fluctuates in the brain. One leading hypothesis at the time was that, when dopamine neurons were activated, the neuromodulator would be released across the brain uniformly. But when Hamid and his colleagues used the dLight sensor to track the molecule’s movements in the striatum of mouse brains — a region that contains many dopamine-responsive neurons — they discovered instead that the neuromodulator was released in waves5. “We were completely floored,” says Hamid, who is now a neuroscientist at the University of Minnesota in Minneapolis. “It really defied our expectations.”
Genetic light bulbs illuminate the brain
Tritsch and his team have found that acetylcholine, too, propagates in waves across the striatum. Using two-colour imaging in live mice with GRAB-DA-based sensors for both acetylcholine and dopamine, the researchers reported in 2023 that, rather than there being a stable, baseline concentration of the two molecules, their levels fluctuate with sub-second kinetics6. “We found signatures of dopamine and acetylcholine on timescales that are much, much faster than we thought was possible,” Tritsch says. “Our next step is to see how that’s influencing the brain on these timescales.”
The sensors are also revealing the secrets of other important neurochemicals. In 2021, a team led by researchers in California and Canada used a GRAB-based sensor to study endocannabinoid signalling in the mouse brain7. And earlier this year, another team used a GPCR sensor, called ntsLight1.1, that responds to the neuropeptide neurotensin, which is involved in feeding behaviour. The aim was to study what drives mice that are fed purely a high-fat diet to start avoiding calorie-rich foods8. Among other things, that analysis implicated a decline in neurotensin levels in a brain region linked with dopamine signalling as a possible cause.
And it’s not only in basic neurobiology that these sensors show promise. For instance, Tian, who is now at the Max Planck Florida Institute for Neuroscience in Jupiter, Florida, and her team have found a use that could help to develop treatments for anxiety. They showed that a sensor targeting neurons affected by the psychedelic drug 2,5-dimethoxy-4-iodoamphetamine can be used to identify other cells that contribute to the drug’s anxiety-reducing effects in mice without producing hallucinogenic effects9. That’s a finding that could support the development of future psychedelics that could have therapeutic benefits without the mind-bending side-effects, the study authors write.
Mixed signals
Genetically encoded sensors do have some important caveats, however.
One key consideration is how these proteins affect the biology of the cells in which they are expressed. Like most proteins, excessive expression can be toxic to neurons, so identifying the best concentration at which to use a sensor is paramount, says Tritsch. “With every batch, we need to know how much to dilute so we can see it, but so it’s not too much that it kills the neurons.”
Why this happens is an open question, but Tritsch and others suggest two possibilities: The GPCRs could be siphoning off molecules that are required for healthy neuronal functioning, or their overexpression could be overextending the cellular machinery required to build these molecules and deliver them to the membrane.