Data reporting
The chosen sample sizes were similar to those used in the field: n = 4–12 samples were used to evaluate the levels of metabolites in serum82,83, cells68,84, tissues68,84,85,86, nematodes87,88,89 and flies90,91,92; n = 4–10 samples to determine OCRs in tissues68,93 and nematodes94,95,96; n = 3–4 samples to determine mRNA levels of a specific gene97,98; n = 2–6 samples to determine the expression levels and phosphorylation levels of a specific protein97; n = 200 worms to determine lifespan99,100,101; n = 60 worms to determine healthspan102,103,104, except n = 10 worms for pharyngeal pumping rates68,105; n = 200 flies, male or female, to determine lifespan106,107,108; n = 60 flies, male or female, to determine healthspan109,110,111; n = 4–8 mice for energy expenditure (EE) and respiratory quotients68; n = 10 mice for hyperinsulinaemic–euglycaemic clamping68,112; n = 5–6 mice for glucose tolerance tests and insulin tolerance tests68; n = 6 mice for body composition68; n = 6 mice for muscle fibre type44,113,114; n = 3 mice for muscle regeneration104,115,116; n = 53–62 mitochondria from 3 mice for muscular mitochondrial content117,118; n = 9–23 mice for running duration68,70; and n = 36–75 mice for grasp strength68. No statistical methods were used to predetermine the sample sizes. All experimental findings were repeated as stated in the figure legends, and all additional replication attempts were successful. For animal experiments, mice, nematodes and flies were housed under the same conditions or places. For cell experiments, cells of each genotype were cultured in the same CO2 incubator and were parallel seeded. Each experiment was designed and performed along with proper controls, and samples for comparison were collected and analysed under the same conditions. Randomization was applied wherever possible. For example, during MS analyses, samples were processed and subjected to MS in random orders. For animal experiments, sex-matched (for mice and flies) and age-matched littermate animals for each genotype were randomly assigned to LCA or vehicle treatments. In cell experiments, cells of each genotype were seeded in parallel and randomly assigned to different treatments. Otherwise, randomization was not performed. For example, when performing IB, samples needed to be loaded in a specific order to generate the final figures. Blinding was applied wherever possible. For example, samples, cages or agar plates or vials during sample collection and processing were labelled as code names that were later revealed by the individual who picked and treated animals or cells but did not participate in sample collection and processing until assessing the outcome. Similarly, during microscopy data collection and statistical analyses, the fields of view were chosen on a random basis and were often performed by different operators, which prevented potentially biased selection for desired phenotypes. Otherwise, blinding was not performed, such as the measurement of OCRs, as different reagents were added for particular reactions.
Mouse strains
WT C57BL/6J mice (000664) were obtained from the Jackson Laboratory. AXINF/F and LAMTOR1F/F mice were generated and validated as previously described97. AMPKA1F/F (014141) and AMPKA2F/F mice (014142) were obtained from the Jackson Laboratory, provided by S. Morrison. AMPKα-MKO mice were generated by crossing AMPKA1/2F/F mice with Mck–Cre mice, as previously described and validated68. Cyp2c cluster KO mice (NM-KO-18019) were purchased from Shanghai Model Organisms Center, and Tgr5 KO mice (S-KO-06069) were from Cyagen. Germ-free C57BL/6J mice were provided by the Laboratory Animal Research Centre of Xiamen University.
The antibiotic-treated mice were generated by exposing C57BL/6J mice to a combination of antibiotics, including 0.5 g l–1 vancomycin, 1 g l–1 metronidazole, 1 g l–1 ampicillin and 1 g l–1 neomycin supplemented in their drinking water (ad libitum) for 5 days. The depletion of gut microbiota was confirmed by testing for the presence of bacterial 16S rDNA in the faeces using PCR. About 100 mg of faeces was freshly collected, followed by extraction of bacterial DNA using a TIANamp Stool DNA kit following the manufacturer’s instructions. 16S rDNA was amplified using Quick-Load Taq 2× master mix on a thermocycler (T100, Bio-Rad) using the universal bacterial 16S rRNA primers 27F and 1492R119 (5′-AGAGTTTGATCCTGGCTCAG-3′ and 5′-TACGGCTACCTTGTTACGACTT-3′) with the following programs: pre-denaturing at 95 °C for 30 s; denaturing at 95 °C for 10 s, annealing at 55 °C for 30 s, then extending at 72 °C for 90 s in each cycle; and final extending at 72 °C for 10 min; cycle number: 29. Mice that tested negative for bacterial 16S rRNA were selected for further experiments.
For analysing AMPK activation, WT mice and AMPKα-MKO mice aged 4 weeks were given LCA for 1 week, and WT mice aged 4 months were subjected to CR for 4 months. For determining rejuvenating effects of LCA or iso-LCA, WT, AMPKα-MKO and Tgr5–/– mice aged 17 months were treated with LCA or iso-LCA for 1 month. For determining rejuvenating effects of CR, WT mice aged 17 months were subjected to CR for 3.5 months. For analysing the pharmacokinetics of LCA, WT mice aged 17 months (aged mice) were treated with LCA for 1 month. For determining the changes in LCA concentrations in serum and tissue, WT and Cyp2c-null mice aged 4 months old were subjected to CR for 4 months, germ-free mice aged 4 months were gavaged with faeces for 1 week and were ad libitum-fed for another 3 weeks, and antibiotic-treated mice aged 4 months were subjected for CR for 3.5 months (except in Fig. 2c, in which mice were subjected to CR for the indicated time durations starting from 4 months old). For determination of the serum metabolome in CR mice, WT mice aged 4 months were subjected to CR for 4 months. For isolating primary hepatocytes and myocytes, WT mice aged 1 month were used.
In this study, the following experiments and measurements were performed using the same group of mice: (1) body weight and body composition, grip strength, EE and running duration; (2) muscle regeneration; (3) mitochondrial content; (4) muscle fibre types; (5) hyperinsulinaemic–euglycaemic clamp tests; (6) intraperitoneal glucose tolerance tests (GTTs) and insulin content; (7) intraperitoneal insulin tolerance tests (ITTs); (8) oral GTTs and GLP-1 measurement; (9) muscular OCRs; (10) muscle weight and muscular mitochondrial mRNA levels; (11) IB; (12) NAD+ levels and mtDNA-to-nDNA ratios; (13) AMP-to-ATP and ADP-to-ATP ratios; and (14) LCA content.
CR, fasting and cardiotoxin treatment of mice
Protocols for all mouse experiments were approved by the Institutional Animal Care and the Animal Committee of Xiamen University (XMULAC20180028 and XMULAC20220050). Mice were housed with free access to water and a standard diet (65% carbohydrate, 11% fat, 24% protein) under specific pathogen-free (SPF) conditions, except for germ-free mice, which were house in a sterile isolator. The light was on from 8:00 to 20:00, with the temperature kept at 21–24 °C and humidity at 40–70%. Male mice were used in this study, except in the experiments shown in Fig. 3c–e and Extended Data Figs. 3a,c,e,f and 4c–e, in which female mice were also used. Littermate controls were used throughout the study.
Mice were individually caged for 1 week before each treatment. For fasting, the diet was withdrawn from the cage at 17:00, and mice were euthanized at specific time points by cervical dislocation. For CR, each mouse was fed 2.5 g of standard diet (approximately 70% of ad libitum food intake for a mouse at 4 months old and older) at 17:00 each day. To perform CR in germ-free conditions, mice were housed in a sterile isolator. Cardiotoxin treatment was performed as previously described104. In brief, mice were anaesthetized with 3% isoflurane in the air using a vaporizer (R540, RWD Life Science). After removal of fur, 50 µl of 20 µM cardiotoxin was intramuscularly injected into the tibialis anterior muscle. Muscles were analysed on day 7 after cardiotoxin injection.
Evaluation of mouse lifespan
Mouse lifespan was determined according to previous reports68,120. In brief, mice were examined every 3–5 days for signs of illness, and the severely moribund ones were killed and recorded. A mouse was considered severely moribund if it showed more than one of the following clinical signs: (1) inability to eat or to drink; (2) severe lethargy, as indicated by lack of response such as reluctance to move when gently prodded with a blunt-tip tweezer; (3) severe balance instability or gait disturbance; (4) rapid weight loss (>3 g) over a period of 1 week; or (5) a severely ulcerated or bleeding tumour. Mice found dead were also noted at each daily inspection.
In Fig. 4g and Supplementary Table 4, cohort 1 started with a total of 679 mice (born around November 2021): 338 males (168 in the vehicle group; 170 in LCA-treatment group) and 341 females (169 in the vehicle group; 172 in the LCA-treatment group). As the experiment progressed, 37 mice were removed (censored) from the study: 25 males (11 vehicle; 14 LCA) and 12 females (8 vehicle; 4 LCA). The reasons for removal included fighting (18 males: 8 vehicle; 10 LCA), paralysis (loss of walking ability; 6 males: 3 vehicle; 3 LCA; and 10 female: 7 vehicle; 3 LCA), and symptoms of gnawing or bruxing (the presence of long, spiral incisors preventing the mouse from eating; 1 male from LCA; and 2 female: 1 vehicle and 1 LCA). Such censored mice were not included in the calculation of lifespan. At the conclusion of the experiment, 14 male mice (6 vehicle; 8 LCA) and 14 female mice (5 vehicle; 9 LCA) were still alive. Cohort 2 started with 687 mice (born around November 2021): 331 males (168 in the vehicle group; 163 in the LCA-treatment group) and 356 females (176 in the vehicle group; 180 in the LCA-treatment group). As the experiment progressed, 56 mice were removed (censored) from the study: 43 males (24 vehicle; 19 LCA) and 13 females (5 vehicle; 8 LCA). The reasons for removal included fighting (35 males: 20 vehicle; 15 LCA) and paralysis (8 males: 4 vehicle; 4 LCA; and 13 females: 5 vehicle; 8 LCA). Such censored mice were not included in the calculation of lifespan. At the conclusion of the experiment, 13 male mice (5 vehicle; 8 LCA) and 13 female mice (5 vehicle; 8 LCA) were still alive. Cohort 3 was started with 622 mice (born around January 2022): 328 males (164 in the vehicle group; 164 in the LCA-treated group) and 294 females (147 in the vehicle group; 147 in the LCA-treated group). As the experiment progressed, 33 mice were removed (censored) from the study: 24 males (12 vehicle; 12 LCA) and 9 females (6 vehicle; 3 LCA). The reasons for removal included fighting (17 males: 10 vehicle; 7 LCA), paralysis (5 males: 2 vehicle; 3 LCA; and 8 females: 5 vehicle; 3 LCA), and symptoms of gnawing or bruxing (2 males from LCA, and 1 female mouse from vehicle). Such censored mice were not included in the calculation of lifespan. At the conclusion of the experiment, 44 male mice (21 vehicle; 23 LCA) and 37 female mice (16 vehicle; 21 LCA) were still alive.
Mouse faecal microbiota transplantation
The faecal transplantation experiment was performed as previously described121,122. In brief, freshly collected faeces from SPF mice were homogenized in sterilized, deoxygenated PBS (prepared by incubating sterilized PBS in an anaerobic incubator supplemented with (v/v) 80% N2, 10% H2 and 10% CO2, for 72 h) at a ratio of 100 mg faeces per ml of PBS, followed by centrifuging for 5 min at 200g. The supernatant was given to antibiotic-treated or germ-free mice by gavage every 2 days during the first week and then every 3 days during weeks 2 and 3, with each mouse receiving 200 μl. Note that the recipient mice received faeces from CR-treated mice, and ad libitum-fed mice were housed separately in different cages or sterile insulators. At the end of week 8, the mice were euthanized for analysis of LCA content.
Formulation and LCA treatment
For cell-based experiments, LCA powder was dissolved in DMSO to a stock concentration of 500 mM, aliquoted and stored at −20 °C. The solution was placed at room temperature for 10 min (until no precipitate was visible) before adding to the culture medium. Note that any freeze–thaw cycle was prohibited to avoid the re-crystallization of LCA (which otherwise formed sheet-like, insoluble crystals) in the stock solution.
For mouse experiments, LCA was coated with (2-hydroxypropyl)-β-cyclodextrin before given to animals. To coat LCA, LCA powder was dissolved in 100 ml methanol to a concentration of 0.01 g ml–1, followed by mixing with 308 ml (2-hydroxypropyl)-β-cyclodextrin solution (by dissolving (2-hydroxypropyl)-β-cyclodextrin in 30% (v/v, in water) methanol to 0.04 g ml–1, followed by 30 min of sonication). The control vehicle was similarly prepared but with no LCA added to the (2-hydroxypropyl)-β-cyclodextrin solution. After evaporating at 50 °C, 90 r.p.m. in a rotary evaporator (Rotavator R-300, Vacuum Pump V-300, BUCHI), the coated powder was stored at 4 °C for no more than 2 weeks and was freshly dissolved in drinking water to 1 g l–1 before given to mice.
For nematode experiments, LCA at desired concentrations was freshly dissolved in DMSO and was added to warm (cooled to approximately 60 °C after autoclaving) nematode growth medium123 (NGM; containing 0.3% (w/v) NaCl, 0.25% (w/v) bacteriological peptone, 1 mM CaCl2, 1 mM MgSO4, 25 mM KH2PO4-K2HPO4, pH 6.0, 0.02% (w/v) streptomycin and 5 μg ml–1 cholesterol). The medium was used to make NGM plates by adding 1.7% (w/v) agar. The plates were stored at 20 °C for no more than 3 days.
For fly experiments, LCA was coated and dissolved in water as for the mouse experiments, and was added to Bloomington Drosophila Stock Center (BDSC) standard cornmeal medium124 (for regular culture), to 2% cornmeal–sugar–yeast (CSY) agar diet (for CR experiments; see ref. 106) or to 3% CSY agar diet (the control diet for CR experiments). The BDSC standard cornmeal medium was prepared as previously described124 but with minor modification. In brief, 60.5 g dry yeast, 35 g soy flour, 255.5 g cornmeal, 20 g agar and 270 ml corn syrup were mixed with 3,500 ml water in a stockpot. The mixture was thoroughly stirred using a long-handled soup spoon and then boiled, during which lumps that formed were pressed out using the back of the spoon. After cooling to approximately 60 °C, 16.8 ml propionic acid was added to the medium followed by stirring with the spoon. The 2% or 3% CSY agar diets were prepared as for the BDSC standard cornmeal medium, except that 175 g cornmeal, 367.5 g sucrose, 70 (for 2% CSY agar diet) or 105 g (for 3% CSY agar diet) dry yeast, 24.5 g of agar, 16.8 ml propionic acid and 3,500 ml water were used. The medium was then dispensed into culture vials (6 ml each). The vials of medium were covered with a single-layer gauze, followed by blowing with the breeze from a fan at room temperature overnight. Next, 100 μl LCA solution (coated with (2-hydroxypropyl)-β-cyclodextrin) at the desired concentration was then layered (added dropwise) onto the surface of the medium of each vial, followed by blowing with the breeze from the fan for another 8 h at room temperature. The vials of medium were kept at 4 °C (for no more than 3 days) before experiment.
Determination of mouse running capacity and grip strength
The maximal running capacity was determined as previously described70,125, but with minor modifications. In brief, mice were trained on a Rodent Treadmill NG (UGO Basile, 47300) for 3 days during the normal light–dark cycle, and tests were performed during the dark period. Before the experiment, mice were fasted for 2 h. The treadmill was set at a 5° incline, and the speed of the treadmill was set to increase in a ramp mode (commencing at a speed of 5 m min–1 followed by an increase to a final speed of 25 m min–1 within 120 min). Mice were considered exhausted and removed from the treadmill following the accumulation of 5 or more shocks (0.1 mA) per min for 2 consecutive minutes. The distances travelled and the endurance were recorded as the running capacity. Note that mice subjected to the test for running capacity were euthanized and no longer used for other experiments.
Grip strength was determined using a grip strength meter (Ugo Basile, 47200) following a previously described protocol104. In brief, the mouse was held by its tail and lowered (landed) until the forelimb or all four limbs grasped the T‐bar connected to a digital force gauge. The mouse was further lowered to such an extent that the body was horizontal to the apparatus, and was then slowly, steadily drawn away from the T‐bar until the forelimb or all four limbs were removed from the bar, which gave rise to the peak force in grams. Each mouse was tested 5 times, with 5-min intervals between measurements. Note that the grip strength of the forelimb and four limbs were measured on different days to prevent interference from muscle tiredness caused by earlier measurements.
Serology, GTT and ITT
GTTs and ITTs were performed as previously described68. Before GTTs and ITTs, mice were individually caged for 1 week before the experiment. For GTTs, mice were fasted for 16 h (17:00 to 9:00) then administered with glucose at 2 g kg–1 (intraperitoneally injected or orally gavaged). For ITTs, mice were fasted for 6 h (8:00 to 14:00), then 0.5 U kg–1 insulin was intraperitoneally injected. Blood glucose was then measured at indicated time points through tail vein bleeding using a OneTouch UltraVue automatic glucometer (LifeScan). Note that GTTs and ITTs were performed using different batches of mice to avoid interference from any stress caused by earlier blood collection.
For measuring insulin levels, approximately 100 μl blood was collected (from the submandibular vein plexus) and was placed at room temperature for 20 min, followed by centrifugation at 3,000g for 10 min at 4 °C. Next, 25 μl of the resultant serum was used to determine insulin levels using a Mouse Ultrasensitive Insulin ELISA kit according to the manufacturer’s instructions. The five-parameter logistic fitted standard curve for calculating the concentrations of insulin was generated from the website of Arigo Biolaboratories (https://www.arigobio.cn/ELISA-calculator/).
For measuring GLP-1 levels, blood samples were collected as for the measurement of insulin, except that the blood collected (approximately 60 μl per sample) was immediately mixed with 2 μl of 50 mM diprotin A for each sample on ice in a K2EDTA spray-coated tube (366420, BD P800 blood collection system). The blood samples were then centrifuged at 3,000g for 10 min at 4 °C, and 10 μl each was used to determine GLP-1 levels using a GLP-1 Multispecies ELISA kit according to the manufacturer’s instructions. The standard curve was generated as for the measurement of insulin.
For measuring free fatty acids, glycerol, β-hydroxybutyrate and glucagon, 1.3 μl, 10 μl, 1 μl and 5 μl of freshly prepared serum from 8-h fasted mice was analysed using a LabAssay NEFA kit, a Free Glycerol Assay kit, a Ketone Body Assay kit or a Mouse Glucagon ELISA Kit, respectively, all following the manufacturer’s instructions.
Hyperinsulinaemic–euglycaemic clamp
Hyperinsulinaemic–euglycaemic clamp testing was performed as previously described112,126,127, but with minor modifications. In brief, mice were anaesthetized with 3% isoflurane in the air using a vaporizer (R540, RWD Life Science). Fur was removed from the incision site and the skin was disinfected with 70% (v/v, in water) ethanol. A small incision located approximately 5 mm superior to the sternum and 5 mm to the right of the vertical midline was made, and the fat and connective tissues beneath the pectoral muscle and surrounding the right jugular vein were gently cleaned by blunt dissection. The cephalad end of the exposed vein was then tightly tied (forming an anterior ligature) by passing a 7-0 silk suture beneath the vein. After loosely tying the caudal end of the vein through another thread of suture (posterior ligature), the vein was inserted using a 19-G needle between the two threads a few millimetres below the anterior ligature. After removing the needle, a catheter (C10PUS-MFV1610, Instech) was inserted into the vein through the needle hole, with the bevel of its tip facing towards the opening, followed by pushing forwards towards the caudal end for approximately 1 cm (until the restraining bead reaching the superior vena cava). The catheter was flushed with 100 μl heparin (200 U ml–1, dissolved in saline) and then anchored by tightening the posterior ligature thread. The catheter was then tunnelled (pulled with eye dressing forceps) beneath the skin from the right jugular incision to the interscapular incision (approximately 5-mm long) on the back. After exteriorizing through the interscapular incision, the catheter was connected to a mouse vascular access button (VABM1BSM-25, Instech) sealed with a protective aluminium cap (VABM1C, Instech) and secured using 6-0 silk suture. After closing the two incisions using the 6-0 silk suture, the catheterized mouse was allowed to recover for 4 days. Mice that lost <4% of their pre-cannulation weight after recovery were used for clamp experiments.
One day before the experiments, a magnetic VAB tether kit (KVABM1T/25, Instech; with its 25-G luer stub (LS25/6, Instech) replaced by a PinPor-to-Tubing connector (PNP3MC/25, Instech), which was connected to a PU tube (VAHBPU-T25, Instech), followed by a 4-way X connector (SCX25, Instech), and three separate luer stubs (LS25, Instech): one for infusing unlabelled glucose, one for [U-13C]glucose and the third for insulin, each connected with a PU tube) was anchored onto a counter-balanced lever arm (SMCLA, Instech) and was flushed with each perfusate by a PHD Ultra programmable syringe pump (HA3000P, Instech) in the following order: unlabelled glucose (20% (m/v) in saline), 100 mU ml–1 insulin and then 0.45 μg μl–1 [U-13C]glucose. The mouse VAB connector on the magnetic VAB tether kit was then connected to the mouse vascular access button on the back of the catheterized mouse after removing the protective aluminium cap. The mice were then fasted for 16 h (starting from 17:00 to 9:00 the following day), and the experiment was performed with a 2-phase protocol consisting of a 90-min equilibration period (t = −90 min to 0 min) and a 120-min experimental period (t = 0 min to 120 min). Next, 0.45 μg μl–1 [U-13C]glucose was given at t = −90 min and was infused at a rate of 30 μg kg–1 min–1 during the remaining time of the experiment. Clamping was begun at t = 0 min with a prime-continuous infusion of insulin (300 mU kg–1 min–1 for 1 min), followed by 25 mU kg–1 min–1 continuous infusion during the remaining time of the experiment. Unlabelled glucose (20% (m/v) in saline) was then infused at 50 mg–1 kg–1 min–1 for 10 min (from t = 0 min to t = 10 min), and the rate was adjusted according to the blood glucose level (maintained at 6–7 mM, during which blood glucose was measured every 10 min from t = 0 to 90 min, and every 5 min from t = 90 to 120 min) thereafter. At t = 0, 90, 100, 110 and 120 min (all at the clamped state), 60 μl blood was taken from the tail vein, and the serum ratios of [U-13C]glucose to unlabelled glucose were determined using an ExionLC AD UPLC system (SCIEX) interfaced with a QTRAP 5500 MS (SCIEX), as described in the section ‘Determination of the serum metabolome’, except that 10 μl serum was used. The resting hepatic glucose output rate (HGP) was calculated by dividing the resting-state infusion rate of [U-13C]glucose with the ratio of [U-13C]glucose to unlabelled glucose, whereas the clamped HGP was calculated by dividing the value of differences between the average, clamp-state infusion rate of the unlabelled glucose and the [U-13C]glucose with the ratio of [U-13C]glucose to unlabelled glucose. The glucose disposal rate during clamping was the sum of the infusion rate of [U-13C]glucose, the infusion rate of unlabelled glucose and the value of clamped HGP.
Determination of body composition
Lean and fat body mass were measured using quantitative magnetic resonance as previously described68, except that male mice were measured using an EchoMRI-100H Analyzer (Echo Medical Systems), whereas female mice were measured using a Live Mouse Body Composition NMR Analyzer (QMR06-090H, Niumag) that also measures free water, one more parameter than the EchoMRI-100H Analyzer. In brief, the system was calibrated to the oil standard before measurement. Mice were individually weighed, inserted into a restrainer tube and immobilized by gently inserting a plunger. The mouse was then positioned so that it curled up like a doughnut, with its head against the end of the tube. The body composition of each mouse was measured with three repeated runs, and the average values were taken for further analysis.
Determination of EE
Mouse EE was determined using a metabolic cage system (Promethion Line, CAB-16-1-EU; Sable Systems International) as previously described68,128. In brief, the system was maintained in a condition identical to that for housing mice. Each metabolic cage in the 16-cage system consisted of a cage with standard bedding, a food hopper and a water bottle connected to load cells for continuous monitoring. To minimize the stress of the new environment, mice were acclimated (by individual housing in the gas-calibrated chamber) for 1 week before data collection. Mice treated with LCA or vehicle control were randomly assigned and housed to prevent systematic errors in measurement. Body weights and fat proportion of mice were determined before and after acclimation, as well as the daily food and water intake. Mice that did not acclimate to the metabolic cage (for example, resisted eating and drinking) were removed from the study. Data acquisition (5-min intervals in each cage) and instrument control were performed using MetaScreen software (v.2.3.15.12, Sable Systems), and raw data were processed using Macro Interpreter (v.2.32, Sable Systems). Ambulatory activity and position were monitored using xyz beam arrays with a beam spacing of 0.25 cm (beam breaks), and the distance walked by the mouse within the cage was calculated accordingly. Respiratory gases were measured using a GA-3 gas analyser (Sable Systems) equipped with a pull-mode, negative-pressure system. Air flow was measured and controlled using a FR-8 (Sable Systems), with a set flow rate of 2,000 ml min–1. Oxygen consumption (VO2) and carbon dioxide production (VCO2) are reported in ml min–1 values. Water vapour was measured continuously, and its dilution effect on O2 and CO2 was compensated mathematically in the analysis stream. EE was calculated using kcal h–1 = 60 × (0.003941 × VO2 + 0.001106 × VCO2) (Weir Equation). Differences in average EE values were analysed by analysis of covariance (ANCOVA) using body weight as the covariate. The respiratory quotient was calculated as VCO2/VO2.
Histology
For haematoxylin and eosin (H&E) staining, muscle tissue was quickly excised, followed by freezing in isopentane (pre-chilled in liquid nitrogen) for 2 min (until they appeared chalky white). The tissue samples were then rapidly transferred to embedding moulds containing OCT compound and were frozen in liquid nitrogen for another 10 min. The embedded tissue samples were then sectioned into 6-μm slices at −20 °C using a CM1950 cryostat (Leica), followed by fixing in 4% paraformaldehyde for 10 min and washing with running water for 2 min at room temperature. The sections were stained in Mayer’s haematoxylin solution for 5 min, followed by washing in running water for 10 min and then stained in eosin Y solution for another 1 min. The stained sections were dehydrated twice in 95% ethanol, 5 min each, twice in anhydrous ethanol, 1 min each, and 2 changes of xylene, 1 min each. The stained sections were mounted with Canada balsam and visualized on an AxioScan 7 scanner (Zeiss). Images were processed and analysed using Zen 3.4 software (Zeiss) and were formatted in Photoshop 2023 software (Adobe).
For immunohistochemistry staining of PAX7, tibialis anterior muscle tissue was excised, embedded and sectioned as for H&E staining. The sections were fixed with 4% paraformaldehyde for 10 min, followed by washing with PBS for 5 min at room temperature. After incubating with PBST (PBS supplemented with 5% Triton X-100) for 10 min, the sections were blocked with BSA solution (PBS containing 5% BSA) for 30 min at room temperature, followed by incubating with a PAX7 antibody (6 μg ml–1, diluted in BSA solution) for 12 h at 4 °C. The sections were then washed with PBS 3 times, 5 min each at room temperature, followed by incubating with Alexa Fluor 488-conjugated, goat anti-mouse IgG1 secondary antibody (1:200 diluted in BSA Solution) for 1 h at room temperature in a dark, humidified chamber. The sections were washed with PBS 3 times, 5 min each at room temperature, followed by incubating with 4% paraformaldehyde for 2 min and then washed with PBS twice, 5 min each at room temperature. The sections were then incubated with the laminin antibody (1:100 diluted in BSA Solution) for 3 h at room temperature in a dark humidified chamber, followed by washing with PBS buffer 3 times, 5 min each at room temperature. The sections were then incubated with Alexa Fluor 594-conjugated, goat anti-rabbit IgG secondary antibody (1:200 diluted in BSA solution) for 1 h at room temperature in a dark humidified chamber, followed by washing with PBS for 3 times, 5 min each at room temperature. Tissue sections were mounted with 90% glycerol and visualized on a LSM980 microscope (Zeiss). Images were processed and analysed using Zen 3.4 software (Zeiss) and formatted in Photoshop 2023 software (Adobe).
Muscle fibre types were determined as previously described44,129, but with minor modifications. In brief, muscle tissue was excised, embedded and sectioned as for H&E staining. The sections were fixed in 4% paraformaldehyde for 10 min and were then washed with PBS for 5 min at room temperature. After incubating with PBST (PBS supplemented with 5% (v/v) Triton X-100) for 10 min, the sections were blocked with BSA solution (PBS containing 5% (m/v) BSA) for 30 min at room temperature. Muscle fibres were stained with antibody against MHCIIb (6 μg ml–1, diluted in BSA solution) overnight at 4 °C, followed by washing with PBS 3 times, 5 min each, at room temperature. The sections were then incubated with Alexa Fluor 488-conjugated, goat anti-mouse IgM antibody (1:200 diluted in BSA Solution) for 1 h at room temperature in a dark humidified chamber, followed by washing with PBS for 3 times, 5 min each, incubated with 4% paraformaldehyde for 2 min, and then washed with PBS twice, 5 min each, all at room temperature. The sections were then incubated with antibody against MHCI (6 μg ml–1, diluted in BSA Solution) for 3 h at room temperature in a dark humidified chamber, followed by washing with PBS buffer 3 times, 5 min each at room temperature, and then incubated with Alexa Fluor 594-conjugated, goat anti-mouse IgG2b antibody (1:200 diluted in BSA solution) for another 1 h at room temperature in a dark humidified chamber, followed by washing with PBS buffer for 3 times, 5 min each at room temperature. After fixing with 4% paraformaldehyde for 2 min and washing with PBS twice, 5 min each at room temperature, the sections were incubated with antibody against MHCIIa (6 μg ml–1, diluted in BSA solution) for 3 h at room temperature in a dark humidified chamber, followed by washing with PBS buffer for 3 times, 5 min each at room temperature, and then incubated in Alexa Fluor 647-conjugated goat anti-mouse IgG1 antibody (1:200 diluted in BSA Solution) for another 1 h at room temperature in a dark humidified chamber, followed by washing with PBS buffer for 3 times, 5 min each at room temperature. Tissue sections were mounted with 90% glycerol and visualized on an LSM980 microscope (Zeiss). Images were processed and analysed using Zen 3.4 software (Zeiss) and formatted in Photoshop 2023 software (Adobe).
Measurements of food and faecal energy content
The energy content in food and faeces was determined using bomb calorimetry as previously described130. In brief, mice were individually housed for 1 week before measurement. The daily food intake (from 17:00 to 17:00 the next day) was continuously measured for 1 week, and the average food consumed by each mouse per day was recorded. Faeces excreted in a day were also collected from 17:00 to 17:00 the next day, pulverized in a ceramic mortar and then lyophilized in a vacuum concentrator (CentriVap Benchtop Centrifugal Vacuum Concentrator, 7310037; Labconco, equipped with a CentriVap −84 °C Cold Trap, 7460037; Labconco, and an EDWARDS nXDS15i pump) at 4 °C for 12 h before measurements using a bomb calorimeter.
Before measurement, an automatic bomb calorimeter (SJLRY-502T, Xinsanjie Instrument and Meter) was standardized by combusting 1 g benzoic acid as a reference (10590.8 J g–1). Next, 1 g of food or faeces was placed on the crucible attached to the bomb head, followed by attaching the fuse wire to the bomb head and adjusting to ensure it touched the top of the food or faeces without touching any other part of the crucible. The loaded bomb head was inserted into the bomb cylinder containing 10 ml distilled water and was screwed on tightly. The bomb was then connected to the oxygen-filling unit and filled with oxygen at a pressure of 2.8–3 MPa for at least 15 s, followed by insertion into the calorimeter bucket. The following steps, including filling the bucket with water, ignition and subsequent combustion, were automatically conducted by the calorimeter. The energy contents were then automatically calculated according to the benzoic acid reference at the end of the combustion.
C.
elegans strains
Nematodes (hermaphrodites) were maintained on NGM plates spread with Escherichia coli OP50 as standard food. All worms were cultured at 20 °C. WT (N2 Bristol) and aak-2 (ok524) strains were obtained from the Caenorhabditis Genetics Center. All mutant strains were outcrossed 6 times to N2 before the experiments. Unless stated otherwise, worms were maintained on NGM plates spread with E. coli OP50 as standard food. The administration of LCA was initiated at the L4 stage.
Evaluation of nematode lifespan and healthspan
To determine the lifespan of nematodes, the worms were first synchronized. Worms were washed off from agar plates with 15 ml M9 buffer (22.1 mM KH2PO4, 46.9 mM Na2HPO4, 85.5 mM NaCl and 1 mM MgSO4) supplemented with 0.05% (v/v) Triton X-100 per plate, followed by centrifugation at 1,000g for 2 min. The worm sediment was suspended with 6 ml M9 buffer containing 50% synchronizing bleaching solution (by mixing 25 ml of NaClO solution (5% active chlorine), 8.3 ml of 25% (w/v) NaOH and 66.7 ml M9 buffer, for a total of 100 ml), followed by vigorous shaking for 2 min and centrifugation for 2 min at 1,000 g. The sediment was washed with 12 ml M9 buffer twice, then suspended with 6 ml M9 buffer followed by rotating at 20 °C, 30 r.p.m. for 12 h. Synchronized worms were cultured to the L4 stage before transfer to desired agar plates for determination of lifespan. Worms were transferred to new plates every 2 days. Live and dead worms were counted during the transfer step. Worms that displayed no movement after gentle touching with a platinum picker were judged dead. Kaplan–Meier curves were generated using Prism 9 (GraphPad Software), and statistical analysis was performed using SPSS 27.0 (IBM).
Pharyngeal pumping rates, assessed as the number of contraction–relaxation cycles of the terminal bulb on the nematode pharynx within 1 min, were determined as previously described131, but with minor modifications. In brief, the synchronized nematodes were cultured to the L4 stage and LCA was administered. The 1-day-old nematodes were then picked and placed on a new NGM plate containing E. coli. After 10 min of incubation at room temperature, the contraction–relaxation cycles of the terminal bulb of each worm were recorded on a stereomicroscope (M165 FC, Leica) through a ×63 objective for a consecutive 4 min using Capture software (v.2021.1.13, Capture Visualisation), and the average contraction–relaxation cycles per min were calculated using Aimersoft Video Editor software (v.3.6.2.0, Aimersoft).
The resistance of nematodes to oxidative stress was determined as previously described102. In brief, synchronized worms were cultured to the L4 stage, after which LCA was administered. After 2 days of LCA treatment, 20 worms were transferred to a NGM plate containing 15 mM FeSO4. Worms were then cultured at 20 °C, during which the number of live and dead worms were counted every 1 h.
D.
melanogaster strains
All flies were cultured at 25 °C and 60% humidity with a 12-h light and dark cycle. Adult flies were cultured in BDSC standard cornmeal medium for regular culture or in 2% (for CR) or 3% (the control, ad libitum-fed group for CR) CSY agar. Larvae and the crossed fly strains were reared on a semi-defined, rich medium, which was prepared as previously described132, but with minor modifications. In brief, 10 g agar, 80 g dry yeast, 20 g yeast extract, 20 g peptone, 30 g sucrose, 60 g glucose, 0.5 g MgSO4·6H2O and 0.5 g CaCl2·6H2O were dissolved in 1,000 ml of di-distilled water and then boiled, followed by cooling to 60 °C. Next, 6 ml propionic acid was added to the medium, and the medium was dispensed into culture vials (6 ml each). The vials of medium were covered with gauze and blown with the breeze of a fan as for the BDSC and CSY diets, and were kept at 4 °C (for no more than 3 days) before experiments.
The WT fly strain (w1118; 3605) and the GAL4-expressing strain (y1 w*; P{Act5C-GAL4-w}E1/CyO; 25374) were obtained from the BDSC. The GAL4-induced, AMPKα RNAi-carrying strain (w1118; P{GD736}v1827; 1827) was obtained from the Vienna Drosophila Resource Center. The w1118; Sp/CyO strain was obtained from the Core Facility of Drosophila Resource and Technology, Chinese Academy of Sciences. To obtain flies with AMPKα knocked down on the w1118 background, a GAL4-expressing strain on the w1118 background (w1118; P{Act5C-GAL4-w}E1/CyO) was first generated by crossing y1 w*; P{Act5C-GAL4-w}E1/CyO males with w1118; Sp/CyO females, followed by crossing the F1 males with straight wings (w1118; P{Act5C-GAL4-w}E1/Sp) with w1118; Sp/CyO females. The GAL4-expressing flies (w1118 background) were then crossed with the AMPKα RNAi-carrying flies, and the F1 offspring with straight wings were the AMPKα knockdown flies (w1118; P{Act5C–GAL4–w}E1/P{GD736}v1827; +/+). The F1 offspring of WT flies crossed with the GAL4-expressing flies (w1118 background), that is, the w1118; P{Act5C–GAL4–w}E1/+; +/+ flies, were used as the control files.
In this study, the following ages of flies were used: (1) for analysing AMPK activation and the pharmacokinetics of LCA, third instar larvae or newly eclosed adults were used; (2) for determining lifespan, adults at day 2 after eclosion were used (for LCA or CR treatment); (3) for determining healthspan, mtDNA-to-nDNA ratios, NAD+ levels and mitochondrial genes expression, adults at day 30 after eclosion (treated with LCA for 28 days starting from 2 days after eclosion) were used.
Evaluation of lifespan and healthspan of flies
Fly lifespan was determined as previously described133, but with minor modifications. Before the experiment, flies were synchronized. Approximately 200 pairs of flies, housed 10 pairs per tube, were cultured in semi-defined, rich medium and allowed to lay eggs for a day. After discarding the parent flies, the embryos were cultured for another 10 days, and the flies that eclosed at day 12 were anaesthetized and collected with CO2 (those that emerged before day 12 were discarded), followed by transfer to BDSC standard cornmeal medium and cultured for another 2 days. The male and female adults were then sorted by briefly anaesthetizing with CO2 on an anaesthetic pad using a homemade feather brush (by attaching the apical region of a vane from the secondary coverts of an adult goose to a plastic balloon stick), and 200 adults of each group and gender were randomly assigned to the BDSC standard cornmeal medium or the CSY medium, with or without LCA, with 20 flies per tube. The flies were transferred to new tubes of medium every 2 days without anaesthesia until the last survivor was dead. During each tube transfer, the sum of dead flies in the old tubes and the dead flies carried to the new tubes were recorded as the numbers of deaths, and the escaped or accidentally killed flies (that is, died within 3 days of same-sex culturing or squeezed by the tube plugs) were censored from the experiments. Kaplan–Meier curves were generated using Prism 9 (GraphPad Software), and statistical analysis was performed using SPSS 27.0 (IBM).
The resistance of flies to oxidative stress was determined as previously described134. In brief, synchronized adults were treated with LCA for 30 days, followed by transfer to vials (20 flies each), each containing a filter paper soaked with 20 mM paraquat or 5% (m/v) H2O2 dissolved or diluted in 5% (w/v, in water) glucose solution. To determine the resistance of flies to cold and heat stress, synchronized adults were treated with LCA for 30 days, followed by transfer to cold (4 °C) or heat (37 °C) stress conditions. To determine the resistance of flies to starvation (food deprivation), flies treated with LCA for 30 days were transferred to vials with culture medium replaced by the same volume of 1.5% agarose to remove the food supply. Dead files were recorded every 2 h until the last survivor was dead.
Quantification of mRNA levels of mitochondrial genes in mice, nematodes and flies
Mice treated with LCA were killed by cervical dislocation, immediately followed by dissecting the gastrocnemius muscle. The muscle tissue was roughly sliced into cubes (edge lengths of approximately 2 mm) and then soaked in RNAprotect tissue reagent (1 ml per 100 mg of tissue) for 24 h at room temperature. The tissue was then incubated in 1 ml TRIzol, followed by 3 rounds of freeze–thaw cycles, and was then homogenized. The homogenate was centrifuged at 12,000g for 15 min at 4 °C, and 900 µl of clear supernatant (not the lipid layer on the top) was transferred to a RNase-free tube. Chloroform (200 µl) was then added to the supernatant, followed by vigorous vortexing for 15 s. After centrifugation at 12,000g for 15 min at 4 °C, 450 µl of the upper aqueous layer was transferred to a RNase-free tube. RNA was then precipitated by adding 450 µl isopropanol, followed by centrifugation at 12,000g for 30 min at 4 °C. The pellet was washed twice with 75% ethanol and once with 100% ethanol, and was dissolved with 20 µl DEPC-treated water. The concentration of RNA was determined using a NanoDrop 2000 spectrophotometer (Thermo). Next, 1 µg RNA was diluted with DEPC-treated water to a final volume of 10 µl, heated at 65 °C for 5 min and chilled on ice immediately. Random Primer mix, Enzyme mix and 5× RT buffer (all from the ReverTra Ace qPCR RT Master Mix) were then added to the RNA solution, followed by incubation at 37 °C for 15 min and then at 98 °C for 5 min on a thermocycler. The reverse-transcribed cDNA was quantified using Maxima SYBR Green/ROX qPCR master mix on a LightCycler 480 II system (Roche) with the following programs: pre-denaturing at 95 °C for 10 min; denaturing at 95 °C for 10 s, then annealing and extending at 65 °C for 30 s in each cycle (determined according to the amplification curves, melting curves and bands on agarose gel of serial pilot reactions (in which a serial annealing temperature was set according to the estimated annealing temperature of each primer pair and same hereafter)) for a total of 45 cycles. Primer pairs for mouse Nd1, Nd2, Nd3, Nd4, Nd4l, Nd5, Nd6, Ndufab1, Cytb, Uqcrc1, Uqcrc2, Atp5f1b, Cox6a1, Atp6, Atp8, Cox1 and Cox3 were generated as previously described135, and others were generated using the Primer-BLAST website (https://www.ncbi.nlm.nih.gov/tools/primer-blast/index.cgi). Primer sequences are as follows: mouse Gapdh, 5′-GACTTCAACAGCAACTCCCAC-3′ and 5′-TCCACCACCCTGTTGCTGTA-3′; mouse Nd1, 5′-TGCACCTACCCTATCACTCA-3′ and 5′-C GGCTCATCCTGATCATAGAATGG-3′; mouse Nd2, 5′-ATACTAGCAATTACTTCTATTTTCATAGGG-3′ and 5′-GAGGGATGGGTTGTAAGGAAG-3′; mouse Nd3, 5′-AAGCAAATCCATATGAATGCGG-3′ and 5′-GCTCATGGTAGTGGAAGTAGAAG-3′; mouse Nd4, 5′-CCTCAGACCCCCTATCCACA-3′ and 5′-GTTTGGTTCCCTCATCGGGT-3′; mouse Nd4l, 5′-CCAACTCCATAAGCTCCATACC-3′ and 5′-GATTTTGGACGTAATCTGTTCCG-3′; mouse Nd5, 5′-ACGAAAATGACCCAGACCTC-3′ and 5′-GAGATGACAAATCCTGCAAAGATG-3′; mouse Nd6, 5′-TGTTGGAGTTATGTTGGAAGGAG-3′ and 5′-CAAAGATCACCCAGCTACTACC-3′; mouse Tfam, 5′-GGTCGCATCCCCTCGTCTAT-3′ and 5′-TTGGGTAGCTGTTCTGTGGAA-3′; mouse Cs, 5′-CTCTACTCACTGCAGCAACCC-3′ and 5′-TTCATGCCTCTCATGCCACC-3′; mouse Ndufs8, 5′-TGGCGGCAACGTACAAGTAT-3′ and 5′-GTAGTTGATGGTGGCAGGCT-3′; mouse Ndufab1, 5′-GGACCGAGTTCTGTATGTCTTG-3′ and 5′-AAACCCAAATTCGTCTTCCATG-3′; mouse Ndufb10, 5′-TGCCAGATTCTTGGGACAAGG-3′ and 5′-GTCGTAGGCCTTCGTCAAGT-3′; mouse Ndufv3, 5′-GTGTGCTCAAAGAGCCCGAG-3′ and 5′-TCAGTGCCGAGGTGACTCT-3′; mouse Ndufa8, 5′-GCGGAGCCTTTCACAGAGTA-3′ and 5′-TCAATCACAGGGTTGGGCTC-3′; mouse Ndufs3, 5′-CTGACTTGACGGCAGTGGAT-3′ and 5′-CATACCAATTGGCCGCGATG-3′; mouse Ndufa9, 5′-TCTGTCAGTGGAGTTGTGGC-3′ and 5′-CCCATCAGACGAAGGTGCAT-3′; mouse Ndufa10, 5′-CAGCGCGTGGGACGAAT-3′ and 5′-ACTCTATGTCGAGGGGCCTT-3′; mouse Sdha, 5′-AGGGTTTAATACTGCATGCCTTA-3′ and 5′-TCATGTAATGGATGGCATCCT-3′; mouse Sdhb, 5′-AGTGCGGACCTATGGTGTTG-3′ and 5′-AGACTTTGCTGAGGTCCGTG-3′; mouse Sdhc, 5′-TGAGACATGTCAGCCGTCAC-3′ and 5′-GGGAGACAGAGGACGGTTTG-3′; mouse Sdhd, 5′-TGGTACCCAGCACATTCACC-3′ and 5′-GGGTGTCCCCATGAACGTAG-3′; mouse Cytb, 5′-CCCACCCCATATTAAACCCG-3′ and 5′-GAGGTATGAAGGAAAGGTATTAGGG-3′; mouse Uqcrc1, 5′-ATCAAGGCACTGTCCAAGG-3′ and 5′-TCATTTTCCTGCATCTCCCG-3′; mouse Uqcrc2, 5′-TTCCAGTGCAGATGTCCAAG-3′ and 5′-CTGTTGAAGGACGGTAGAAGG-3′; mouse Atp5f1b, 5′-CCGTGAGGGCAATGATTTATAC-3′ and 5′-GTCAAACCAGTCAGAGCTACC-3′ mouse Cox6a1, 5′-GTTCGTTGCCTACCCTCAC-3′ and 5′-TCTCTTTACTCATCTTCATAGCCG-3′; mouse Atp6, 5′-TCCCAATCGTTGTAGCCATC-3′ and 5′-TGTTGGAAAGAATGGAGTCGG-3′; mouse Atp8, 5′-GCCACAACTAGATACATCAACATG-3′ and 5′-TGGTTGTTAGTGATTTTGGTGAAG-3′; mouse Atp5f1a, 5′-CATTGGTGATGGTATTGCGC-3′ and 5′-TCCCAAACACGACAACTCC-3′; mouse Cox1, 5′-CCCAGATATAGCATTCCCACG-3′ and 5′-ACTGTTCATCCTGTTCCTGC-3′; mouse Cox2, 5′-TCTACAAGACGCCACATCCC-3′ and 5′-ACGGGGTTGTTGATTTCGTCT-3′; mouse Cox3, 5′-CGTGAAGGAACCTACCAAGG-3′ and 5′-CGCTCAGAAGAATCCTGCAA-3′; mouse Cox5b, 5′-AGCTTCAGGCACCAAGGAAG-3′ and 5′-TGGGGCACCAGCTTGTAATG-3′. The mRNA level was then calculated using the comparative ΔΔct method with LightCycler software (v.96 1.1, Roche; same hereafter for all qPCR experiments).
Nematodes at the L4 stage treated with LCA for 1 day were used for the analysis of mitochondrial gene expression. Around 1,000 worms were collected into 15 ml M9 buffer containing 0.05% Triton X-100 (v/v), followed by centrifugation for 2 min at 1,000g. The sediment was then washed with 1 ml M9 buffer twice and then lysed with 1 ml TRIzol. Worms were then frozen in liquid nitrogen, thawed at room temperature and then the freeze–thaw cycle was repeated for another 2 times. The worm lysates were then placed at room temperature for 5 min, mixed with 0.2 ml of chloroform, followed by vigorous shaking for 15 s. After centrifugation at 12,000g for 15 min at 4 °C, 450 µl of the upper aqueous layer was transferred to a RNase-free tube. RNA was then precipitated by adding 450 µl isopropanol, followed by centrifugation at 12,000g for 30 min at 4 °C. The pellet was washed twice with 75% ethanol and once with 100% ethanol, and was dissolved with 20 µl of DEPC-treated water. The concentration of RNA was determined using a NanoDrop 2000 spectrophotometer (Thermo). Next, 1 µg RNA was diluted with DEPC-treated water to a final volume of 10 µl, heated at 65 °C for 5 min and chilled on ice immediately. Random Primer mix, Enzyme mix and 5× RT buffer (all from the ReverTra Ace qPCR RT Master Mix) were then added to the RNA solution, followed by incubation at 37 °C for 15 min and then at 98 °C for 5 min on a thermocycler. The reverse-transcribed cDNA was quantified using Maxima SYBR Green/ROX qPCR master mix on a LightCycler 480 II system (Roche) with the following programs: pre-denaturing at 95 °C for 10 min; denaturing at 95 °C for 10 s, then annealing and extending at 65 °C for 30 s in each cycle, for a total of 45 cycles. Primer pairs used for qPCR are as previously described136,137, except that C. elegans ctb-1 was designed using the Primer-BLAST website. Primer sequence are as follows: C. elegans ama-1, 5′-GACATTTGGCACTGCTTTGT-3′ and 5′-ACGATTGATTCCATGTCTCG-3′; C. elegans nuo-6, 5′-CTGCCAGGACATGAATACAATCTGAG-3′ and 5′-GCTATGAGGATCGTATTCACGACG-3′; C. elegans nuaf-1, 5′-GAGACA TAACGAGGCTCGTGTTG-3′ and 5′-GAAGCCTTCTTTCCAATCACTATCG-3′; C. elegans sdha-1, 5′-TTACCAGCGTGCTTTCGGAG-3′ and 5′-AGGGTGTGGAGAAGAGAATGACC-3′; C. elegans sdhb-1, 5′-GCTGAACGTGATCGTCTTGATG-3′ and 5′-GTAGGATGGGCATGACGTGG-3′; C. elegans cyc-2.1, 5′-CGGA GTTATCGGACGTACATCAG-3′ and 5′-GTCTCGCGGGTCCAGACG-3′; C. elegans isp-1, 5′-GCAGAAAGATGAATGGTCCGTTG-3′ and 5′-ATCCGTGACAAGGGCAGTAATAAC-3′; C. elegans cco-1, 5′-GCTGGAGATGATCGTTACGAG-3′ and 5′-GCATCCAATGATTCTGAAGTCG-3′; C. elegans cco-2, 5′-GTGATACCGTCTACGCCTACATTG-3′ and 5′-GCTCTGGCACGAAGAATTCTG-3′; C. elegans atp-3, 5′-GTCCTCGACCCAACTCTCAAG-3′ and 5′-GTCCAAGGAAG TTTCCAGTCTC-3′; C. elegans nduo-1, 5′-AGCGTCATTTATTGGGAAGAAGAC-3′ and 5′-AAGCTTGTGCTAATCCCATAAATGT-3′; C. elegans nduo-2, 5′-TCTT TGTAGAGGAGGTCTATTACA-3′ and 5′-ATGTTAAAAACCACATTAGCCCA-3′; C. elegans nduo-4, 5′-GCACACGGTTATACATCTACACTTATG-3′ and 5′-GATGTATGATAAAATTCACCAATAAGG-3′; C. elegans nduo-5, 5′-AGATGAGATTTATTGGGTATTTCTAG-3′ and 5′-CACCTAGACGATTAGTTAATGCTG-3′; C. elegans ctc-1, 5′-GCAGCAGGGTTAAGATCTATCTTAG-3′ and 5′-CTGTTACAAATACAGTTCAAACAAAT-3′; C. elegans ctc-2, 5′-GTAGTTTATTGTTGGGAGTTTTAGTG-3′ and 5′-CACAATAATTCACCAAACTGATACTC-3′; C. elegans atp-6, 5′-TGCTGCTGTAGCGTGATTAAG-3′ and 5′-ACTGTTAAAGCAAGTGGACGAG-3′; C. elegans ctb-1, 5′-TGGTGTTACAGGGGCAACAT-3′ and 5′-TGGCCTCATTATAGGGTCAGC-3′.
Drosophila adults treated with LCA for 30 days were used to determine the expression of mitochondrial genes. For each sample, 20 adults were used. The adults were anaesthetized, transferred to a 1.5-ml Eppendorf tube, followed by quickly freezing in liquid nitrogen and then homogenized using a pellet pestle (Z359963-1EA, Sigma). The homogenate was then lysed in 1 ml TRIzol for 5 min at room temperature, followed by centrifugation at 12,000g for 15 min at 4 °C. Next, 900 μl supernatant (without the lipid layer) was transferred to a RNase-free tube and mixed with 200 μl of chloroform. After vigorous vortexing for 15 s, the mixture was centrifuged at 12,000g for 15 min at 4 °C, and 450 μl of the upper aqueous layer was transferred to a RNase-free tube. RNA was then precipitated by adding 450 μl isopropanol, followed by centrifugation at 12,000g for 30 min at 4 °C. The pellet was washed twice with 75% (v/v, in water) ethanol, and was dissolved with 20 μl of DEPC-treated water. The concentration of RNA was determined using a NanoDrop 2000 spectrophotometer (Thermo). Next, 1 µg RNA was diluted with DEPC-treated water to a final volume of 10 µl, heated at 65 °C for 5 min and chilled on ice immediately. Random Primer mix, Enzyme mix and 5× RT buffer (all from the ReverTra Ace qPCR RT Master Mix) were then added to the RNA solution, followed by incubation at 37 °C for 15 min and then at 98 °C for 5 min on a thermocycler. The reverse-transcribed cDNA was quantified using Maxima SYBR Green/ROX qPCR master mix on a LightCycler 480 II system (Roche) with the following programs: pre-denaturing at 95 °C for 5 min; denaturing at 95 °C for 10 s, then annealing at 60 °C for 20 s, and then extending at 72 °C for 20 s in each cycle, for a total of 40 cycles. Primer pairs used for qPCR are as previously described138, and are listed as follows: D. melanogaster CG9172, 5′-CGTGGCTGCGATAGGATAAT-3′3′ and 5′-ACCACATCTGGAGCGTCTTC-3′; D. melanogaster CG9762, 5′-AGTCACCGCATTGGTTCTCT-3′3′ and 5′-GAGATGGGGTGCTTCTCGTA-3′; D. melanogaster CG17856, 5′-ACCTTTCCATGACCAAGACG-3′ and 5′-CTCCATTCCTCACGCTCTTC-3′; D. melanogaster CG18809, 5′-AAGTGAAGACGCCCAATGAGA-3′ and 5′-GCCAGGTACAACGACCAGAAG-3′; D. melanogaster CG5389, 5′-ATGGCTACAGCATGTGCAAG-3′ and 5′-GACAGGGAGGCATGAAGGTA-3′; D. melanogaster Act5C, 5′-GCAGCAACTTCTTCGTCACA-3′ and 5′-CATCAGCCAGCAGTCGTCTA-3′.
Analysis of mtDNA copy numbers in mice, nematodes and flies
Mouse mtDNA copy numbers were determined as previously described68. In brief, mouse tissue DNA was extracted using a Biospin tissue genomic DNA extraction kit (BioFlux) following the manufacturer’s instruction, but with minor modifications. In brief, mice treated with LCA were killed by cervical dislocation, quickly followed by dissecting the gastrocnemius muscle. The muscle tissue was then ground in liquid nitrogen on a ceramic mortar. Next, 50 mg ground tissue was transferred to a 1.5-ml Eppendorf tube, followed by addition of 600 µl of FL buffer and 10 µl of PK solution containing 2 µl of 100 mg ml–1 RNase A. The mixture was then incubated at 56 °C for 15 min, followed by centrifugation at 12,000g for 3 min. Then 500 µl supernatant was transferred to a 2-ml Eppendorf tube, followed by mixing with 700 µl binding buffer and 300 µl absolute ethanol. The mixture was then loaded onto a Spin column and was centrifuged at 10,000g for 1 min. The flow through was discarded, and 500 µl PW buffer was added to the Spin column, followed by centrifugation at 10,000g for 30 s. Next, 600 µl washing buffer was added to the spin column followed by centrifugation at 10,000g for 30 s, and this process was repeated once. The Spin column was then centrifuged for 1 min at 10,000g to completely remove the washing buffer, and the DNA on the column was eluted with 100 µl of Elution buffer (added to the Spin column, followed by incubation at room temperature for 5 min and then centrifuged at 12,000g for 1 min). Total DNA was quantified using Maxima SYBR Green/ROX qPCR master mix on a LightCycler 480 II system (Roche) with the following programs: 70 ng DNA was pre-denatured at 95 °C for 10 min, and then subjected to PCR for a total of 45 cycles: denaturing at 95 °C for 10 s, annealing and extending at 65 °C for 30 s in each cycle. Primer pairs used for qPCR are as previously described139 (mouse Hk2, 5′-GCCAGCCTCTCCTGATTTTAGTGT-3′ and 5′-GGGAACACAAAAGACCTCTTCTGG-3′; mouse Nd1, 5′-CTAGCAGAAACAAACCGGGC-3′ and 5′-CCGGCTGCGTATTCTACGTT-3′).
Nematode mtDNA copy numbers were determined from worm lysates as previously described68. In brief, 30 synchronized early L4 worms were collected and were lysed with 10 μl of worm lysis buffer (50 mM HEPES, pH7.4, 1 mM EGTA, 1 mM MgCl2, 100 mM KCl, 10% (v/v) glycerol, 0.05% (v/v) NP-40, 0.5 mM DTT and protease inhibitor cocktail). The worm lysate was frozen at −80 °C overnight, followed by incubating at 65 °C for 1 h and 95 °C for 15 min. Nematode DNA was then quantified using Maxima SYBR Green/ROX qPCR master mix on a LightCycler 480 II system (Roche) with the following programs: pre-denaturing at 95 °C for 10 min and then for a total of 45 cycles of denaturing at 95 °C for 10 s, and annealing and extending at 65 °C for 30 s in each cycle. Primer pairs used for qPCR are designed as previously described103 (C. elegans nd-1, 5′-AGCGTCATTTATTGGGAAGAAGAC-3′ and 5′-AAGCTTGTGCTAATCCCATAAATGT-3′; C. elegans act-3, 5′-TGCGACATTGATATCCGTAAGG-3′ and 5′-GGTGGTTCCTCCGGAAAGAA-3′).
Drosophila DNA copy numbers were determined as previously described140, but with minor modifications. In brief, 20 anaesthetized adults were homogenized in 100 μl Fly Lysis buffer (75 mM NaCl, 25 mM EDTA and 25 mM HEPES, pH7.5) containing proteinase K (100 μg ml–1). The homogenate was then frozen at −80 °C for 12 h, followed by incubating at 65 °C for 1 h and 95 °C for another 15 min. Fly DNA was then quantified using Maxima SYBR Green/ROX qPCR master mix on a LightCycler 480 II system (Roche) with the following programs: pre-denaturing at 95 °C for 5 min and then for a total of 40 cycles of denaturing at 95 °C for 10 s, and annealing 60 °C for 20 s and extending at 72 °C for 20 s in each cycle. Primer pairs used for qPCR are as previously described140 (D. melanogaster 16S rRNA, 5′-TCGTCCAACCATTCATTCCA-3′ and 5′-TGGCCGCAGTATTTTGACTG-3′; D. melanogaster RpL32, 5′-AGGCCCAAGATCGTGAAGAA-3′ and 5′-TGTGCACCAGGAACTTCTTGAA-3′).
Determining muscle atrophy markers in mice
Muscle atrophy markers were determined as previously described141, but with minor modifications. In brief, mice treated with LCA were killed by cervical dislocation, followed by quickly dissecting the gastrocnemius muscle. Muscle tissue was sliced into cubes (edge lengths of approximately 2 mm) and then soaked in RNAprotect Tissue reagent (1 ml per 100 mg of tissue) for 24 h at room temperature. The tissue was then incubated in 1 ml TRIzol, followed by 3 rounds of freeze–thaw cycles and was then homogenized. The homogenate was centrifuged at 12,000g for 15 min at 4 °C, and 900 µl of the clear supernatant (without the lipid layer) was transferred to a RNase-free tube. Chloroform (200 µl) was added to the supernatant, followed by a vigorous vortex for 15 s. RNA was then purified and was transcribed to cDNA as described in the section ‘Quantification of mRNA levels of mitochondrial genes in mice, nematodes and flies’. The reverse-transcribed cDNA was quantified using Maxima SYBR Green/ROX qPCR master mix on a LightCycler 480 II system (Roche) with the following programs: pre-denaturing at 95 °C for 10 min; denaturing at 95 °C for 10 s, then annealing and extending at 65 °C for 30 s in each cycle for a total of 45 cycles. Primer sequences are as previously described141 (mouse Fbxo32, 5′-TAGTAAGGCTGTTGGAGCTGATAG-3′ and 5′-CTGCACCAGTGTGCATAAGG-3′; mouse Trim63, 5′-CATCTTCCAGGCTGCGAATC-3′; and 5′-ACTGGAGCACTCCTGCTTGT-3′; mouse Gapdh, 5′-TTCACCACCATGGAGAAGGC-3′ and 5′-CCCTTTTGGCTCCACCCT-3′).
Primary hepatocytes and myocytes
Mouse primary hepatocytes were isolated using a modified two-step perfusion method with liver perfusion medium and liver digest buffer as previously described70. Before isolation of hepatocytes, mice were first anaesthetized, followed by inserting a 0.72 × 19 mm intravenous catheter into the postcava. After cutting off the portal vein, mice were perfused with 50 ml liver perfusion medium at a rate of 5 ml min–1, followed by 50 ml liver digest buffer at a rate of 2.5 ml min–1. The digested liver was then briefly rinsed by PBS and then dismembered by gently tearing apart the Glisson’s capsule with two sterilized, needle-pointed tweezers on a 6-cm dish containing 3 ml PBS. The dispersed cells were mixed with 10 ml ice-cold William’s medium E plus 10% FBS, and were filtered through a 100-μm cell strainer (352360; Falcon). Cells were then centrifuged at 50g at 4 °C for 2 min, then washed twice with 10 ml ice-cold William’s medium E plus 10% FBS. Cells were then immediately plated (at 60–70% confluence) in collagen-coated 6-well plates in William’s medium E plus 10% FBS, 100 IU penicillin and 100 mg ml–1 streptomycin, and were maintained at 37 °C in a humidified incubator containing 5% CO2. After 4 h of attachment, the medium was replaced with fresh William’s medium E with 1% (w/v) BSA for another 12 h before further use.
Mouse primary myocytes were isolated as previously described142. In brief, mice were killed by cervical dislocation, and hindlimb muscles from both legs were excised. Tissue samples were minced and digested in a collagenase B–dispase–CaCl2 solution for 1.5 h at 37 °C in a shaking bath. DMEM supplemented with 10% FBS was then added to the digested tissue samples, and the mixtures were gently triturated, followed by loading onto a 70-μm strainer filter (352350; Falcon). Cell suspensions were then centrifuged at 1,000g for 5 min, and the pellets were resuspended in a growth medium (Ham’s F-10 medium supplemented with 20% FBS and 2.5 ng ml–1 bFGF). Cells were then plated on collagen-coated dishes (354456, Corning) at 60–70% confluence.
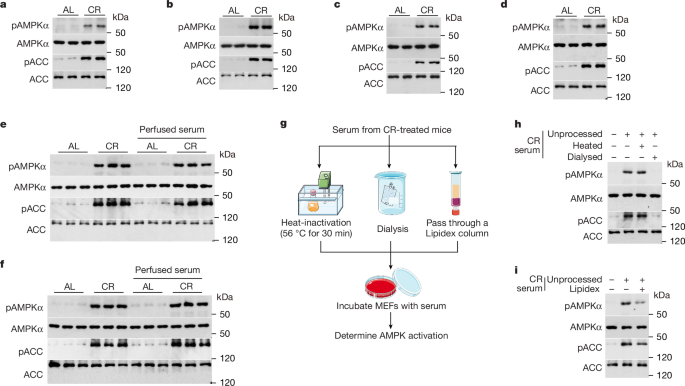
Determination of the serum metabolome
For measuring the serum metabolome, blood samples from CR-treated and ad libitum-fed mice were collected at 15:00. The samples were incubated at room temperature for 10 min and then centrifuged at 3,000g at 4 °C for another 10 min. The supernatants were serum samples, which were prepared on the same day of blood collection. For heat-inactivation, serum was incubated at 56 °C for 30 min in a water bath. For dialysis, serum was loaded into a D-Tube Dialyzer Maxi (with molecular weight cut-offs from 3.5 to 14 kDa; 71508) and dialysed in a beaker containing 2 l PBS at 4 °C for 24 h on a magnetic stirrer. The PBS was refreshed every 4 h.
Polar metabolites were determined by HPLC–MS, GC–MS and CE–MS as previously described68,84,143, but with minor modifications144. For HPLC–MS and CE–MS, 100 μl serum was instantly mixed with 1 ml pre-cooled methanol containing IS1 (50 µM l-methionine sulfone, 50 µM d-campher-10-sulfonic acid, dissolved in water; 1:500 (v/v) added to the methanol and used to standardize the metabolite intensity and to adjust the migration time), then mixed with 1 ml chloroform and 400 μl water (containing 4 μg ml–1 [U-13C]-glutamine), followed by 20 s of vortexing. After centrifugation at 15,000g for 15 min at 4 °C, the supernatant (aqueous phase) was then divided into 3 portions: (1) 200 μl for HPLC–MS analysis; (2) 200 μl for CE–MS analysis on anion mode; and (3) 200 μl for CE–MS analysis on cation mode. Portion (1) was then lyophilized in a vacuum concentrator (CentriVap Benchtop Centrifugal Vacuum Concentrator (c7310037; Labconco) equipped with a CentriVap −84 °C Cold Trap (7460037; Labconco) and an EDWARDS nXDS15i pump) at 4 °C for 12 h and then dissolved in 50 μl of 50% (v/v, in water) acetonitrile, followed by centrifugation at 15,000g for another 30 min at 4 °C. Next, 20 µl of the supernatant was loaded into an injection vial (5182-0714, Agilent Technologies; with an insert (HM-1270, Zhejiang Hamag Technology)) equipped with a snap cap (HM-2076, Zhejiang Hamag Technology), and 2 μl of the supernatant was injected into a HILIC column (ZICpHILIC, 5 μm, 2.1 mm × 100 mm, PN: 1.50462.0001, Millipore) on an ExionLC AD UPLC system (SCIEX), which was interfaced with a QTRAP 5500 MS (SCIEX). The mobile phase consisted of 15 mmol l–1 ammonium acetate containing 3 ml l–1 ammonium hydroxide (>28%, v/v) in LC–MS grade water (mobile phase A), and LC–MS grade 90% (v/v) acetonitrile in LC–MS grade water (mobile phase B), and was run at a flow rate of 0.2 ml min–1. The HPLC gradient elution program was 95% B held for 2 min, then to 45% B for 13 min, held for 3 min, and then back to 95% B for 4 min. Each sample was analysed on both positive and negative modes on the HPLC–MS instrument. The mass spectrometer was run on a Turbo V ion source with spray voltages of −4,500 V (negative mode) and 5,500 V (positive mode), source temperature at 550 °C, gas no. 1 at 50 psi, gas no. 2 at 55 psi and curtain gas at 40 psi. Metabolites were measured using the multiple reactions monitoring mode (MRM), and declustering potentials and collision energies were optimized using analytical standards. Data were collected using Analyst software (v.1.7.1, SCIEX), and the relative amounts of metabolites were analysed using MultiQuant software (v.3.0.3, SCIEX). Portions (2) and (3) of the supernatant were filtered through a 5-kDa cut-off filter (OD003C34, PALL) by centrifuging at 12,000g for 3 h at 4 °C. The filtered aqueous phase was then lyophilized at 4 °C and then re-dissolved in 100 μl water containing IS2 (50 µM 3-aminopyrrolidine dihydrochloride, 50 µM N,N-diethyl-2-phenylacetamide, 50 µM trimesic acid and 50 µM 2-naphtol-3,6-disulfonic acid disodium salt, dissolved in methanol; used to adjust the migration time; 1:200 for portion (2) or 1:400 for portion (3)). Next, 20 μl of re-dissolved portion (2) and portion (3) solutions was loaded into an injection vial (9301-0978, Agilent Technologies; equipped with a snap cap (5042-6491, Agilent Technologies)). Before CE–MS analysis, the fused-silica capillary (TSP050375, i.d. 50 µm × 80 cm; Polymicro Technologies) was installed in a CE–MS cassette (G1603A; Agilent Technologies) on a 7100 CE system (Agilent Technologies). For anion mode, the capillary was pre-conditioned with 1 M NaOH for 0.5 h, flushed with di-distilled water for 2 h and then washed with anion conditioning buffer (25 mM ammonium acetate and 75 mM diammonium hydrogen phosphate, pH 8.5) for 1 h, followed by balancing with anion running buffer (50 mM ammonium acetate, pH 8.5; freshly prepared) for another 2 h. The capillary was then washed again using anion conditioning buffer for 5 min, followed by injection of the samples at a pressure of 50 mbar for 25 s, and then separation with a constant voltage at −30 kV for another 40 min in the anion running buffer. Sheath liquid (0.1 μM hexakis(1H, 1H, 3H-tetrafluoropropoxy)phosphazine, 10 μM ammonium trifluoroacetate, dissolved in methanol–water (50% v/v); freshly prepared) was flowed at 1 ml min–1 through a 1:100 flow splitter (1260 Infinity II; Agilent Technologies; actual flow rate to the MS: 10 μl min–1) throughout each run. The parameters of 6545 MS (Agilent Technologies) were set as follows: ion source, dual AJS ESI; polarity, negative; nozzle voltage, 2,000 V; fragmentor voltage, 110 V; skimmer voltage, 50 V; OCT RFV, 500 V; drying gas (N2) flow rate, 7 l min–1; drying gas (N2) temperature, 300 °C; nebulizer gas pressure, 8 psig; sheath gas temperature, 125 °C; sheath gas (N2) flow rate, 4 l min–1; capillary voltage (applied onto the sprayer), 3,500 V; reference (lock) masses, m/z 1,033.988109 for hexakis(1H, 1H, 3H-tetrafluoropropoxy)phosphazine and m/z 112.985587 for trifluoroacetic acid; scanning range, 50–1,100 m/z; and scanning rate, 1.5 spectra s–1. For cation mode, the capillary was pre-conditioned with 1 M NaOH for 30 min, followed by flushing with di-distilled water for 2 h and then cation running buffer (1 mol l–1 formic acid, freshly prepared) for another 2 h. Samples were separated as for the anion mode, except that cation running buffer was used, the capillary voltage was set to 3,500 V and the fragmentor voltage was 80 V. Data were collected using MassHunter LC–MS acquisition 10.1.48 (Agilent Technologies) and were processed using Qualitative Analysis B.06.00 (Agilent Technologies).
To analyse polar metabolites by GC–MS68, 50 μl of each serum sample was instantly mixed with 200 μl methanol containing 40 μg ml–1 tridecanoic acid and 10 µg ml–1 myristic-d27 acid as internal standards, followed by 20 s of vortexing and 30 min of incubation at −20 °C. The mixture was then centrifuged at 15,000g for 15 min at 4 °C, and 200 μl of supernatant (aqueous phase) was lyophilized at 4 °C for 24 h. The lyophilized sample was then vortexed for 1 min after mixing with 50 μl of freshly prepared methoxyamine hydrochloride (20 mg ml–1 in pyridine), followed by incubation at 4 °C for 1 h. The mixture was sonicated at 0 °C by bathing in an ice slurry for 10 min, and was then incubated at 37 °C for 1.5 h, followed by mixing with 50 μl MSTFA and incubated at 37 °C for 1 h. Before subjecting to GC–MS, samples were centrifuged at 15,000g for 10 min, and 60 μl of each supernatant was loaded into an injection vial (5182-0714, Agilent; with an insert (HM-1270, Zhejiang Hamag Technology)) equipped with a snap cap (HM-0722, Zhejiang Hamag Technology). GC was performed on a HP-5MS column (30 m × 0.25 mm i.d., 0.25 μm film thickness) using a GC/MSD instrument (7890-5977B, Agilent Technologies). The injector temperature was set to 260 °C. The column oven temperature was first held at 70 °C for 2 min, then increased to 180 °C at the rate of 7 °C min–1, then to 250 °C at the rate of 5 °C min–1, then to 310 °C at the rate of 25 °C min–1, at which it was held for 15 min. The MSD transfer temperature was 280 °C. The MS quadrupole and source temperature were maintained at 150 °C and 230 °C, respectively. Data were collected using MassHunter GC–MS Acquisition software (v.B.07.04.2260, Agilent Technologies) and were analysed using GC–MS MassHunter Workstation Qualitative Analysis software (v.10.1.733.0, Agilent Technologies).
Quantitative lipidomics was performed using stable isotope dilution methods145,146. In brief, lipids were extracted from 50 µl of serum using previously described method147, but with modifications. Serum samples were mixed with 750 µl chloroform–methanol–MilliQ water (same hereafter for lipidomics) (3:6:1 v/v/v). After incubating at 1,500 r.p.m. for 1 h at 4 °C on a ThermoMixer C (Eppendorf), 350 µl water and 250 µl chloroform were added to the mixture to induce phase separation. After transferring the organic phase to a clean Eppendorf tube, the remaining lipid in the mixture was extracted again through the addition of another 450 μl chloroform. The organic phase obtained from the two rounds of extraction was pooled and lyophilized using a SpeedVac Vacuum Concentrator (Genevac) under OH mode. Samples were then dissolved in 100 µl of chloroform–methanol (1:1) (v/v) containing an internal standard cocktail (see ref. 147). Lipidomics analyses were conducted at LipidALL Technologies using a Nexera 20-AD HPLC (Shimadzu) coupled with QTRAP 6500 PLUS MS (SCIEX), as previously described146. For polar lipids, normal phase (NP) HPLC was performed using a TUP-HB silica column (i.d. 150 × 2.1 mm, 3 µm; Tuplabs), and the gradient elution program was as follows: 2% B (chloroform–methanol–ammonium hydroxide–water, mixed at 55:39:0.5:5.5 (v/v); with mobile phase A: chloroform–methanol–ammonium hydroxide, 89.5:10:0.5 (v/v)) held for 2 min, followed by 3 incremental increases: (1) to 35% B at the 3rd min, (2) to 55% B at the 5th min, and (3) to 85% B at the 6th min; and then maintained at 85% B for 1 min, followed by increasing to 100% B within 0.2 min and maintained for another 3.8 min, and finally decreased to 2% B within 0.5 min. The column was equilibrated at 2% B for 4.5 min between each run. Polar lipids were qualified by three separate injections under ESI mode, with two injections in positive mode at two separate dilutions (for phosphatidylcholine (PC), lysophosphatidylcholine (LPC), sphingomyelin (SM), ceramide (Cer), glucosylceramide (GluCer), lactosylceramide (LacCer) and sphingosine (Sph); to guarantee that all polar lipids detected fall within the linear ranges of intensities), and one injection in negative mode (for phosphatidylethanolamine (PE), phosphatidylglycerol (PG), phosphatidylinositol (PI), phosphatidic acid (PA), phosphatidylserine (PS), bis(monoacylglycero)phosphate (BMP), also known as lysobisphosphatidic acid), cardiolipin (CL), ganglioside (GM3), saccharolipids (SL), free fatty acid (FFA), lysophosphatidylethanolamine (LPE), lysophosphatidylinositol (LPI), lysophosphatidic acid (LPA), lysophosphatidylserines (LPS) and PC with fatty acyl-specific transitions). MS source parameters were set as follows: curtain gas flow (CUR), 20; temperature (TEM), 400 °C; ion source gas 1 (GS1), 20; and ion source gas 2 (GS2), 20. MRM transitions were set up for the quantitative analysis of polar lipids. Each polar lipid species was quantified by referencing to spiked internal standards, including d9-PC32:0(16:0/16:0), d9-PC36:1p(18:0p/18:1), d7-PE33:1(15:0/18:1), d9-PE36:1p(18:0p/18:1), d31-PS(d31-16:0/18:1), d7-PA33:1(15:0/18:1), d7-PG33:1(15:0/18:1), d7-PI33:1(15:0/18:1), C17-SL, Cer d18:1/15:0-d7, C12:0 Cer-1-P, d9-SM d18:1/18:1, C8-GluCer, C8-GalCer, d3-LacCer d18:1/16:0 Gb3 d18:1/17:0, d7-LPC18:1, d7-LPE18:1, C17-LPI, C17-LPA, C17-LPS, C17-LPG, d17:1 Sph, d17:1 S1P (Avanti Polar Lipids), GM3-d18:1/18:0-d3 (Matreya), d31-16:0 (Sigma) and d8-20:4 (Cayman Chemicals). For neutral lipids (TAGs and DAGs), a Kinetex-C18 column (i.d. 4.6 × 100 mm, 2.6 µm; Phenomenex) and an isocratic mobile phase containing chloroform–methanol–0.1 M ammonium acetate 100:100:4 (v/v/v) at a flow rate of 300 µl min–1 for 10 min were used. MS source parameters were set as described above, and ESI-positive mode was used. Levels of short-chain, medium-chain and long-chain TAGs were quantified by referencing to spiked internal standards of TAG(14:0)3-d5, TAG(16:0)3-d5 and TAG(18:0)3-d5 (CDN isotopes), whereas DAGs d5-DAG17:0/17:0 and d5-DAG18:1/18:1 (Avanti Polar Lipids) were used. For free Cho and total cholesteryl esters, the method involving atmospheric pressure chemical ionization in positive mode on a 1260 Infinity II HPLC (Agilent Technologies) coupled to a QTRAP 5500 MS (SCIEX), as established previously148, was used, during which lipids were separated on an Eclipse XDB C18 5-μm column (i.d. 150 × 4.6 mm; Agilent Technologies) using an isocratic mobile phase comprising chloroform–methanol (1:1 v/v) at a flow rate of 700 μl min–1, and the MS source was set as follows: CUR 20; temperature, 500 °C; GS1, 45; and GS2 35. MS data were acquired and analysed using Analyst 1.6.3 software (SCIEX). During the analysis, quality control samples, pooled from analysis samples, were inserted into the sample queue across every ten biological samples.
Measurement of adenylates and NAD+
ATP, ADP, AMP and NAD+ from cells, tissues or flies were analysed by CE–MS as described in the section ‘Determination of the serum metabolome’, except that cells collected from a 10-cm dish (60–70% confluence), 100 mg of liver or muscle tissue dissected by freeze clamping, or 20 anaesthetized adult flies were used. Before CE–MS analysis, cells were rinsed with 20 ml of 5% (m/v) mannitol solution (dissolved in water) and instantly frozen in liquid nitrogen. Cells were then lysed with 1 ml methanol containing IS1 (1:500 dilution) and were scraped from the dish. For analysis of metabolites in the liver and muscle, mice were anaesthetized after indicated treatments. The tissue was then quickly excised by freeze clamping and then ground in 1 ml methanol with IS1. For analysis of metabolites in flies, anaesthetized flies were ground in 1 ml methanol with IS1 after freezing by liquid nitrogen. The lysate was then mixed with 1 ml chloroform and 400 μl water by 20 s of vortexing. After centrifugation at 15,000g for 15 min at 4 °C, 420 μl of the aqueous phase was collected, filtrated, lyophilized, dissolved and subjected to CE–MS analysis in the negative mode as described in the section ‘Determination of the serum metabolome’. Data were collected using MassHunter LC–MS acquisition 10.1.48 (Agilent Technologies) and were processed using Qualitative Analysis B.06.00 (Agilent Technologies). Levels of AMP, ADP, ATP and NAD+ were measured using full scan mode with m/z values of 346.0558, 426.0221, 505.9885 and 662.1019, respectively. Note that a portion of ADP and ATP could lose one phosphate group during in-source fragmentation, thus leaving the same m/z ratios as AMP and ADP, and should be corrected according to their different retention times in the capillary. Therefore, the total amount of ADP is the sum of the latter peak of the m/z 346.0558 spectrogram and the former peak of the m/z 426.0221 spectrogram, and the same is applied for ATP. Note that the retention time of each metabolite (and IS1 and IS2) may vary between each run and can be adjusted using isotope-labelled standards (dissolved in individual cell or tissue lysates) run between each sample.
Levels of ATP, ADP, AMP and NAD+ in nematodes were analysed by HPLC–MS as described in the section ‘Determination of the serum metabolome’, except that 150 nematodes maintained on NGM plates (with or without 50 mM) for 48 h were used. Nematodes were washed with ice-cold M9 buffer containing Triton X-100. Bacteria were removed by quickly spinning down the slurry at 100g for 5 s. Nematodes were then instantly lysed in 1 ml methanol then mixed with 1 ml chloroform and 400 μl water (containing 4 µg ml–1 [U-13C]-glutamine), followed by 20 s of vortexing. After centrifugation at 15,000g for another 15 min at 4 °C, 800 μl of the aqueous phase was collected, lyophilized in a vacuum concentrator at 4 °C and then dissolved in 30 μl of 50% (v/v, in water) acetonitrile. Metabolites were determined by HPLC–MS in the negative mode as described in the section ‘Determination of the serum metabolome’. The following transitions (Q1/Q3) were used for monitoring each compound: 505.9/158.9 and 505.9/408.0 for ATP; 425.9/133.9, 425.9/158.8 and 425.9/328.0 for ADP; 345.9/79.9, 345.9/96.9 and 345.9/133.9 for AMP; 662.0/540.1 for NAD+; and 149.9/114 for [U-13C]-glutamine. Data were collected using Analyst software (v.1.7.1, SCIEX), and the relative amounts of metabolites were analysed using MultiQuant software (v.3.0.3, SCIEX). Similar to CE–MS analysis, a portion of ADP and ATP could lose one or two phosphate groups during in-source-fragmentation, thus leaving the same m/z ratios as AMP and ADP, which was corrected according to their different retention times in the column.
Determination of bile acid concentrations
To measure bile acid concentrations, MEFs (60–70% in 10-cm dish, rinsed with PBS and trypsinized), tissues (50 mg of liver or muscle tissues collected from each mouse after anaesthetizing and blood-draining), nematodes (1,000 nematodes at L4 stage, washed with M9 buffer containing Triton X-100 for 3 times and frozen in liquid nitrogen) and flies (20 anaesthetized adults washed with PBS for 3 times and frozen in liquid nitrogen) were rinsed in PBS for 5 times, each in a fresh Eppendorf tube, or serum (50 μl collected from each mouse) was vigorously mixed (for serum) or homogenized (for others) with 1 ml of 80% methanol (v/v, in water) containing 100 μg l–1 CA-d5 and 4 µg ml–1 [U-13C]-glutamine as internal standards. After centrifugation at 15,000g for 15 min at 4 °C, 30 µl of the supernatant was loaded into an injection vial (5182-0714, Agilent Technologies; with an insert (HM-1270, Zhejiang Hamag Technology)) equipped with a snap cap (HM-2076, Zhejiang Hamag Technology), and 2 μl of supernatant was injected into an ACQUITY HSS T3 column (Waters). Measurement was performed on a QTRAP 6500 Plus MS (SCIEX) connected to an ACQUITY I-class UPLC system (Waters). The mobile phase consisted of 10 mM ammonium formate containing 0.005% formic acid (v/v) in LC–MS grade water (mobile phase A) and LC–MS grade acetonitrile (mobile phase B) run at a flow rate of 0.4 ml min–1. The HPLC gradient was as follows: 30% B for 1 min, then to 100% B at the 10th min, hold for 2 min, and then back to 30% B, hold for another 3 min. The mass spectrometer was run on a Turbo V ion source and running in negative mode at a spray voltage of −5,500 V, with source temperature at 550 °C, gas no. 1 at 50 psi, gas no. 2 at 60 psi, curtain gas at 35 psi, and collision gas at medium. Compounds were measured using the MRM mode, and declustering potentials and collision energies were optimized using analytical standards. The following transitions (Q1/Q3) were used for monitoring each compound: 391.2/345.2 for deoxycholate; 407.2/345.2 for CA; 448.4/74.0 for glycochenodeoxycholate; 464.2/74.0 for glycocholate; 498.3/80.0 for taurodeoxycholate; 391.2/391.2 for ursodeoxycholate; 375.2/375.2 for LCA; 391.2/373.2 for CDCA; 482.2/80.0 for taurolithocholate; 498.2/79.9 for tauroursodeoxycholate; 448.4/74.0 for glycodeoxycholate; 514.3/79.9 for taurocholate; 498.3/80.0 for taurochenodeoxycholate; 407.3/387.3 for α-muricholate; 407.3/371.1 for β-muricholate; 407.3/405.3 for ω-muricholate; 514.3/123.9 for tauro-α-muricholate; 514.3/79.8 for tauro-β-muricholate; 149.9/114 for [U-13C]-glutamine; and 412.3/348.3 for CA-d5. For quantification of LCA, the LCA-d4 dissolved in individual lysates was used to generate corresponding standard curves by plotting the area ratios of labelled LCA (areas of LCA divided by the two ISs) against the added or actual concentrations of labelled LCA. The concentrations of LCA were estimated according to standard curves. The average volume and density were 2,000 μm3 (ref. 85) and 1.1 g ml–1 (ref. 149), respectively, for MEFs; 3,500 μm3 and 1.06 g ml–1, respectively, for mouse myocytes150,151; and 1 × 106 μm3 and 1.07 g ml–1, respectively, for nematodes152,153. Data were collected using Analyst software (v.1.6.3, SCIEX), and the relative amounts of metabolites were analysed using MultiQuant software (v.3.0.2, SCIEX).
Reagents
The following antibodies were purchased from Cell Signaling Technology: rabbit anti-phospho-AMPKα-Thr172 (2535, RRID: AB_331250; 1:1,000 for IB); anti-AMPKα (2532, RRID: AB_330331; 1:1,000 for IB); anti-phospho-ACC-Ser79 (3661, RRID: AB_330337; 1:1,000 for IB); anti-ACC (3662, RRID: AB_2219400; 1:1,000 for IB); anti-GAPDH (5174, RRID: AB_10622025; 1:1,000 for IB); anti-phospho-p70 S6K-S389 (9234, RRID: AB_2269803; 1:1,000 for IB); anti-p70 S6K (2708, RRID: AB_390722; 1:1,000 for IB); anti-TFEB (83010, 1:100 for immunofluorescence (IF)); anti-phospho-RPA32/RPA2-S8 (54762, RRID: AB_2799471; 1:1,000 for IB); anti-RPA32/RPA2 (35869, RRID: AB_2799086; 1:1,000 for IB); and anti-AXIN1 (2074, RRID: AB_2062419; 1:1,000 for IB). Rabbit anti-tubulin (10068-1-AP; RRID: AB_2303998; 1:1,000 for IB) was purchased from Proteintech. The following antibodies were purchased from Abcam: mouse anti-total OXPHOS (ab110413, RRID: AB_2629281; 1:5,000 for IB); rabbit anti-laminin (ab11575, RRID: AB_298179; 1:200 for IF); anti-gamma H2A.X (ab11174, RRID: AB_297813; 1:1000 for IB); and anti-UCP1 (ab10983, RRID: AB_2241462; 1:1,000 for IB). The following antibodies were purchased from Developmental Studies Hybridoma Bank: mouse anti-eMHC (BF-G6, RRID: AB_10571455; 1:100 for IHC); anti-Pax7 (Pax-7, RRID: AB_2299243; 1:100 for IHC); anti-MHCIIa (SC71, RRID: AB_2147165; 1:100 for IHC); anti-MHCIIb (BF-F3, RRID: AB_2266724; 1:100 for IHC); and anti-MHCI (C6B12, RRID: AB_528351; 1:100 for IHC). Mouse anti β-acting (A5316, RRID: AB_476743; 1:1,000 for IB) was purchased from Sigma. The following antibodies were purchased from Thermo: goat anti-Mouse IgM (heavy chain) cross-adsorbed secondary antibody, Alexa Fluor 488 (A-21042, RRID: AB_2535711; 1:200 for IHC); goat anti-mouse IgG2b cross-adsorbed secondary antibody, Alexa Fluor 594 (A-21145, RRID: AB_2535781; 1:200 for IHC); goat anti-mouse IgG1 cross-adsorbed secondary antibody, Alexa Fluor 647 (A-21240, RRID: AB_2535809; 1:200 for IHC); goat anti-mouse IgG1 cross-adsorbed secondary antibody, Alexa Fluor 488 (A-21121, RRID: AB_2535764; 1:200 for IHC); goat anti-rabbit IgG (H+L) cross-adsorbed secondary antibody, Alexa Fluor 594 (A-11012, RRID: AB_2534079; 1:200 for IHC); and goat anti-rabbit IgG (H+L) highly cross-adsorbed secondary antibody, Alexa Fluor 488 (A-11034, RRID: AB_2576217; 1:100 for IF). The horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG (115-035-003, RRID: AB_10015289; 1:5,000 dilution for IB) and goat anti-rabbit IgG (111-035-003, RRID: AB_2313567; 1:5,000 dilution for IB) antibodies were purchased from Jackson ImmunoResearch.
Information on supplier and catalogue numbers of metabolites for screening are listed in Supplementary Table 1. The following reagents were purchased from Sigma (catalogue numbers in parentheses): DMSO (D2650), LCA (L6250), ferulic acid (PHR1791), (2-hydroxypropyl)-β-cyclodextrin (C0926), NaCl (S7653), CaCl2 (C5670), MgSO4 (M2643), H2O2 (H1009), KH2PO4 (P5655), K2HPO4 (P9666), streptomycin (85886), cholesterol (C3045), agar (A1296), propionic acid (P5561), sucrose (S7903), glucose (G7021), haematoxylin solution (51275), heparin (H3149), 2-methylbutane (isopentane; M32631), paraformaldehyde (158127), eosin Y-solution (318906), methanol (646377), ethanol (459836), chloroform (C7559), PBS (P5493), Triton X-100 (T9284), xylene (534056), d-mannitol (M4125), Canada balsam (C1795), BSA (A2153), glycerol (G5516), Na2HPO4 (S7907), sodium hypochlorite solution (NaClO; 239305), NaOH (S8045), iron(II) sulfate heptahydrate (FeSO4; F8633), isopropanol (34863), diethylpyrocarbonate (DEPC)-treated water (693520), paraquat (36541), HEPES (H4034), EDTA (E6758), EGTA (E3889), MgCl2 (M8266), KCl (P9333), IGEPAL CA-630 (NP-40, I8896, for IF only), IGEPAL CA-630 (NP-40; 3021), dithiothreitol (DTT; 43815), hexadimethrine bromide (polybrene; H9268), collagenase B (11088831001), dispase II (4942078001), proteinase K (P6556), agarose (A9539), l-methionine sulfone (M0876), d-campher-10-sulfonic acid (1087520), acetonitrile (34888), ammonium acetate (73594), ammonium hydroxide solution (338818), 3-aminopyrrolidine dihydrochloride (404624), N,N-diethyl-2-phenylacetamide (384011), trimesic acid (482749), diammonium hydrogen phosphate (1012070500), ammonium trifluoroacetate (56865), formic acid (5.43804), tridecanoic acid (T0502), myristic-d27 acid (68698), methoxyamine hydrochloride (89803), hexane (34859), pyridine (270970), MSTFA (M-132), cholic acid-2,2,3,4,4-d5 (CA-d5; 614106), ammonium formate (70221), lithocholic acid-2,2,4,4-d4 (LCA-d4; 589349), Trizma base (Tris; T1503), sodium pyrophosphate (P8135), β-glycerophosphate (50020), SDS (436143), sodium deoxycholate (S1827), glutaraldehyde solution (G5882), glycine (G8898), K3Fe(CN)6 (455946), thiocarbonohydrazide (223220), Pb(NO3)2 (203580), acetone (534064), sodium citrate (71497), PD98059 (P215), FCCP (C2920), sodium azide (NaN3; S2002), gentamycin (345814), collagenase A (11088793001), oligomycin A (75351), cardiotoxin (217503), Tween-20 (P9416), NaH2PO4 (S8282), formaldehyde solution (formalin; F8775), ampicillin (A9518), metronidazole (M3761), neomycin (N6386), benzoic acid (NIST39J), Nile Red (72485), and Ketone Body Assay kit (MAK134). Vancomycin (V32969) was purchased from InvivoChem. CA (HY-N0324), CDCA (HY-76847), TUDCA (HY-19696), TCA (HY-B1788), 1-methyladenosine (HY-113081) and diprotin A (HY-111174) were purchased from MedChemExpress. Rapamycin (S1039), 4-methyl-2-oxovaleric acid (S2987), mandelic acid (S9003) and methylmalonic acid (S3683) were purchased from Selleck. 3-Hydroxynaphthalene-2,7-disulfonic acid disodium salt (2-naphtol-3,6-disulfonic acid disodium salt; H949580) was purchased from Toronto Research Chemicals. Hexakis(1H,1H,3H-perfluoropropoxy)phosphazene (hexakis(1H,1H,3H-tetrafluoropropoxy)phosphazine; sc-263379) was purchased from Santa Cruz Biotechnology. Protease inhibitor cocktail (70221) was purchased from Roche. Seahorse XF base medium (103334) was purchased from Agilent Technologies. RNAprotect Tissue Reagent (76106) was purchased from Qiagen. OsO4 (18465) and uranyl acetate (19481) were purchased from Tedpella. SPI Low Viscosity “Spurr” Kit (02680-AB) was purchased from Structure Probe. Isolithocholic acid (iso-LCA; 700195) was purchased from Avanti Polar Lipids. 3-Oxo-5β-cholanoic acid (3-oxo-LCA; HY-125801), allolithocholic acid (allo-LCA; HY-143712), isoallolithocholic acid (isoallo-LCA; HY-B0172A) and 3-oxoallo-LCA (customized) were purchased from MCE. Free Glycerol Assay kit (ab65337) and Glycogen Assay kit (ab65620) were purchased from Abcam. Bacteriological peptone (LP0037) and yeast extract (LP0021) were purchased from Oxoid. Insulin (Novolin R) was purchased from Novo Nordisk. Quick-Load Taq 2× Master Mix (M0271), NEBNext Poly(A) mRNA Magnetic Isolation Module (E7490), and NEBNext Ultra RNA Library Prep kit for Illumina (E7530) were purchased from NEB. AMPure XP Beads (63881) was purchased from Beckman Coulter. TruSeq PE Cluster kit v3-cBot-HS kit (PE-401-3001) was purchased from Illumina. Biospin Tissue Genomic DNA Extraction kit (BSC04M1) was purchased from BioFlux. The following reagents were purchased from Thermo: DMEM-high glucose (12800082), Liver Perfusion Medium (17701038), Liver Digest Medium (17703034), William’s E Medium (12551032), Ham’s F-10 medium (11550043), basic fibroblast growth factor (bFGF; 13256-029), Schneider’s Drosophila Medium (21720024), DMEM containing HEPES (21063029), DMEM without phenol (31053028), MEM non-essential amino acids solution (11140050), GlutaMAX (35050061), sodium pyruvate (11360070), Maxima SYBR Green/ROX qPCR Master Mix (K0223), Trypan Blue Stain (T10282), FBS (10099141C), Fluo-4-AM (F14217), ProLong Diamond antifade mountant (P36970), ProLong Live Antifade reagent (P36975), penicillin–streptomycin (15140163), Prestained Protein MW Marker (26612), BCA Protein Assay kit (A55865), Maxima SYBR Green/ROX qPCR master mix (K0223), TRIzol (15596018), and GLP-1 Multispecies ELISA Kit (BMS2194). ReverTra Ace qPCR RT master mix with gDNA remover (FSQ-301) was purchased from Toyobo. WesternBright ECL and peroxide solutions (210414-73) were purchased from Advansta. Dry yeast (FLY804020F) and cornmeal (FLY801020) were purchased from LabScientific. Soy flour (62116) was purchased from Genesee. Light corn syrup was purchased from Karo. O.C.T. Compound (4583) was purchased from Sakura. Mouse Ultrasensitive Insulin ELISA kit (80-INSMSU-E10) was purchased from ALPCO. LabAssay Triglyceride Kit (LABTRIG-M1) and LabAssay NEFA kit (LABNEFA-M1) were purchased from Wako. Mouse Glucagon ELISA Kit (81518) was purchased from Crystal Chem. [U-13C]-glucose (CLM-1396-PK) and [U-13C]-glutamine (CLM-1822-H-PK) were purchased from Cambridge Isotope Laboratories.
Cell lines
In this study, no cell line used is on the list of known misidentified cell lines maintained by the International Cell Line Authentication Committee (https://iclac.org/databases/cross-contaminations/). HEK293T cells (CRL-3216) and Drosophila Schneider 2 (S2) cells (CRL-1963) were purchased from the American Type Culture Collection. MEFs were established by introducing SV40 T antigen through lentivirus into cultured primary embryonic cells from mouse litters as previously described97. HEK293T cells and MEFs were maintained in DMEM supplemented with 10% FBS, 100 IU penicillin and 100 mg ml–1 streptomycin at 37 °C in a humidified incubator containing 5% CO2. S2 cells were cultured in Schneider’s Drosophila medium supplemented with 10% heat-inactivated FBS, 100 IU penicillin and 100 mg ml–1 streptomycin at 37 °C in a humidified incubator containing 5% CO2. All cell lines were verified to be free of mycoplasma contamination. HEK293T cells and MEFs were authenticated by STR sequencing, and Drosophila S2 cells by species identification using DNA-barcode assay, performed by Immocell Biotechnology.
The Tgr5 gene was deleted from MEFs using the CRISPR–Cas9 system. Oligonucleotides were annealed to their complements containing the cloning tag aaac and inserted into the back-to-back BsmB I restriction sites of the lentiCRISPRv2 vector. The sequence for sgRNA is as follows: 5′-TAGTGGTGGGCGACGCTCAT-3′ and 5′-ATGAGCGTCGCCCACCACTA-3′ (1), 5′-GGCTGCGCAAGTGGCGGTCC-3′ and 5′-GGACCGCCACTTGCGCAGCC-3′ (2). The constructs were then subjected to lentivirus packaging using HEK293T cells that were transfected with 2 µg of DNA in Lipofectamine 2000 transfection reagent per well of a 6-well plate. At 30 h after transfection, medium (DMEM supplemented with 10% FBS and MEM non-essential amino acids; approximately 2 ml) was collected and centrifuged at 5,000g for 3 min at room temperature. The supernatant (virus) was mixed with 10 μg ml–1 polybrene and was added to MEFs (cultured to 15% confluence), followed by centrifuging at 3,000g for 30 min at room temperature (spinfection). Cells were incubated with the virus for 72 h. When cells were approaching confluence, they were single-cell sorted into 96-well dishes. Clones were expanded and evaluated for knockout status by sequencing.
IB analysis
To analyse pAMPKα and pACC levels in HEK293T cells, MEFs, S2 cells, mouse primary hepatocytes and primary myocytes, cells grown to 70–80% confluence in a well of a 6-well dish were lysed with 250 μl ice-cold Triton lysis buffer (20 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% (v/v) Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate and protease inhibitor cocktail). The lysates were then centrifuged at 20,000g for 10 min at 4 °C, and an equal volume of 2× SDS sample buffer was added into the supernatant. Samples were then boiled for 10 min and then directly subjected to IB. To analyse of pAMPKα and pACC levels in muscle and liver tissues, mice were anaesthetized after indicated treatments. For CR-treated and LCA-treated mice, tissue samples were collected at 8:00 unless stated otherwise. Freeze-clamped tissue samples were immediately lysed with ice-cold Triton lysis buffer (10 μl mg–1 tissue weight for liver, and 5 μl mg–1 tissue weight for muscle), followed by homogenization and centrifugation as described above. The lysates were then mixed with 2× SDS sample buffer, boiled and subjected to IB. To analyse pAMPKα and pACC levels in flies, 20 adults or third instar larvae were lysed with 200 μl ice-cold RIPA buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate and protease inhibitor cocktail) containing 0.1% SDS, followed by homogenization and centrifugation as described above. The lysates were then mixed with 5× SDS sample buffer, boiled and subjected to IB. To analyse pAMPKα and pACC levels in nematodes, 150 nematodes cultured on NGM plates were collected for each sample. Worms were quickly washed with ice-cold M9 buffer containing Triton X-100 and were lysed with 150 μl ice-cold lysis buffer. The lysates were then mixed with 5× SDS sample buffer, followed by homogenization and centrifugation as described above, and then boiled before being subjected to IB. Precautions in sample preparation were taken for determination of AMPK activation, as previously described154. In brief, for cultured cells, the culture medium was rapidly removed and the cells were immediately lysed with ice-cold lysis buffer or frozen in liquid nitrogen. Tissue samples were freeze-clamped on anaesthetized mice, followed by immediate transfer to liquid nitrogen, as any ischaemia would lead to activation of CaMKK2, which strongly activates AMPK, to such an extent that it could overshadow the lysosomal pathway-mediated activation of AMPK. When homogenizing the tissue, it was directly transferred to ice-cold lysis buffer from liquid nitrogen and homogenized immediately without allowing it to thaw to room temperature. In addition, any exposure to a poor medium such as PBS would cause a higher background of AMPK. All samples were subjected to IB on the same day of preparation, and any freeze–thaw cycles were avoided.
For IB, SDS–PAGE gels were prepared in-house as previously described155. The thickness of the gels used in this study was 1.0 mm. Samples of less than 10 μl were loaded into wells, and electrophoresis was run at 100 V (by PowerPac HC High-Current Power Supply, Bio-Rad) in a Mini-PROTEAN Tetra electrophoresis cell (Bio-Rad). In this study, all samples were resolved on 8% resolving gels, except those for OXPHOS proteins, which were on 15% gels (prepared as those for 8%, except that a final concentration of 15% acrylamide–Bis was added to the resolving gel solution), and β-actin which was on 10% gels. The resolved proteins were then transferred to a PVDF membrane (0.45 μm, IPVH00010, Merck) as previously described155. The PVDF membrane was then blocked with 5% (w/v) BSA (for all antibodies against phosphorylated proteins) or 5% (w/v) non-fat milk (for all antibodies against total proteins) dissolved in TBST for 2 h on an orbital shaker at 60 r.p.m. at room temperature, followed by rinsing with TBST twice, for 5 min each. The PVDF membrane was then incubated with the desired primary antibody overnight at 4 °C on an orbital shaker at 60 r.p.m., followed by rinsing with TBST 3 times, 5 min each at room temperature, and then secondary antibodies for 3 h at room temperature with gentle shaking. The secondary antibody was then removed, and the PVDF membrane was further washed with TBST 3 times, 5 min each at room temperature. The PVDF membrane was incubated in an ECL mixture (by mixing equal volumes of ECL solution and peroxide solution for 5 min), then life with medical X-ray film (Fujifilm). The films were then developed using X-OMAT MX developer (Carestream) and X-OMAT MX Fixer and Replenisher solutions (Carestream) on a medical X-ray processor (Carestream) using developer (model 002, Carestream). The developed films were scanned using a Perfection V850 Pro scanner (Epson) with Epson Scan software (v.3.9.3.4), and were cropped using Photoshop 2023 software (Adobe). Levels of total proteins and phosphorylated proteins were analysed on separate gels, and representative immunoblots are shown. The band intensities on developed films were quantified using ImageJ software (v.1.8.0, National Institutes of Health Freeware) and formatted using Illustrator 2022 (Adobe). Uncropped immunoblots are shown in Supplementary Fig. 1.
Confocal microscopy
For determining the localization of TFEB, MEFs grown to 80% confluence on coverslips in 6-well dishes were fixed for 20 min with 4% (v/v) formaldehyde in PBS at room temperature. The coverslips were rinsed twice with PBS and permeabilized with 0.5% (v/v) NP-40 in PBS for 15 min at room temperature. After rinsing twice with PBS, the sections were blocked with PBS containing 5% BSA for 30 min at room temperature. The coverslips were then incubated with anti-TFEB antibodies (1:100, diluted in PBS) for 8 h at room temperature, followed by rinsing 3 times with 1 ml of PBS. Cells were then incubated with Alexa Fluor 488-conjugated, goat anti-rabbit IgG secondary antibody (1:100, diluted in PBS) for 2 h at room temperature, and at 37 °C for another 30 min in the dark. Cells were washed another 4 times each with 1 ml of PBS and then mounted on slides using ProLong Diamond antifade mountant. Confocal microscopy images were taken using a STELLARIS 8 FALCON (Leica) systems equipped with HyD SMD detectors and a HC PL APO CS2 ×63/1.40 OIL objective (Leica). All parameters were kept unchanged between imaging. Images were taken and analysed using LAS X Software (Leica) and formatted using Photoshop 2023 software (Adobe).
Transmission electron microscopy imaging
Transmission electron microscopy (TEM) imaging was performed based on the in situ embedding and sectioning method as previously described156, but with minor modifications. In brief, the tibialis anterior muscle was quickly excised and sliced to around 1 × 1 × 5 mm cubes, followed by rapid immersion in 1 ml of 2.5% (v/v) glutaraldehyde solution (freshly prepared by diluting 25% (v/v) glutaraldehyde in 0.1 M phosphate buffer (by mixing 0.2 M Na2HPO4 with 0.2 M NaH2PO4 (both dissolved in water, and adjusted pH to 7.4) at a ratio of 81:19, and then diluted with equal volume of water) at 4 °C for 12 h. Muscle samples were then washed with 1 ml of 0.1 M phosphate buffer 3 times at 4 °C, 20 min each, followed by staining with 1% (w/v) OsO4 solution (in 0.1 M phosphate buffer, supplemented with 1.5% K3Fe(CN)6) at 4 °C for 2 h, and then washed by for 5 times, 10 min each with ice-cold di-distilled water. Muscle samples were stained in ice-cold 2% (w/v, in water) uranyl acetate solution for 12 h at 4 °C in the dark, and were then washed 4 times, 10 min each, with ice-cold water. Dehydration was then performed by sequentially incubating muscles in the following solutions: 30, 50 and 70% (v/v) ethanol (in water), each for 12 min at 4 °C, followed by incubating in 90, 100 and 100% (v/v) ethanol (in water), each for 12 min at room temperature, and then in acetone twice at room temperature. Muscle samples were then quickly submerged in acetone–Spurr resin (3:1; the Spurr resin was prepared by mixing 15 g NSA, 7.3 g DER 736, 7.5 g ERL 4206 with 320 μl DMAE, all supplied in the SPI Low Viscosity ‘Spurr’ kits, for 1.5 h at room temperature) mixture at room temperature for a 1-h incubation, and then in acetone/resin (1:1) mixture at room temperature for 2 h, followed by acetone–resin (1:3) at room temperature for 2 h, and finally 100% resin at room temperature for 12 h. The resin was then completely drained, and the tissue samples were baked in a hot-wind drying oven at 70 °C. After baking for 24 h, the tissues were then sectioned into 70-nm slices on an EM UC7 ultramicrotome (Leica) after cooling down to room temperature. Sections were then stained with lead citrate solution (by dissolving 1.33 g of Pb(NO3)2 and 1.76 g of sodium citrate in 42 ml of di-distilled water, followed by addition of 8 ml of 1 M NaOH) for 5 min at room temperature before imaging using an AMT-XR81DIR camera on an electron microscope (HT-7800, Hitachi) with TEM system control software (v.01.20, Hitachi). The area of mitochondria was quantified using ImageJ software (v.1.8.0, National Institutes of Health Freeware).
Determination of intracellular Ca2+ levels
Intracellular Ca2+ levels in MEFs treated with LCA were determined as previously described70. In brief, cells were loaded with 5 μM (final concentration) Fluo-4-AM for 30 min, then washed twice with PBS and incubated in fresh, desired medium for another 30 min. Before imaging, ProLong Live Antifade reagent was added to the medium. During imaging, live cells were kept at 37 °C, 5% CO2 in a humidified incubation chamber (Incubator PM S1; Zeiss). Images were taken on an LSM980 microscope (Zeiss). Images were processed and analysed using Zen 3.4 software (Zeiss) and formatted in Photoshop 2023 software (Adobe).
Determination of OCRs
The OCR of nematodes was measured as previously described157. In brief, nematodes were washed with M9 buffer 3 times. Next, 15–25 nematodes were suspended in 200 μl M9 buffer and were added into a well on a 96-well Seahorse XF Cell Culture microplate. The measurement was performed in a Seahorse XFe96 analyzer (Agilent Technologies) at 20 °C following the manufacturer’s instructions, with a Seahorse XFe96 sensor cartridge (Agilent Technologies) pre-equilibrated in Seahorse XF Calibrant solution in a CO2-free incubator at 37 °C overnight. Concentrations of respiratory chain inhibitors used during the assay were FCCP at 10 μM and sodium azide at 40 mM. At the end of the assay, the exact number of nematodes in each well was determined using a Cell Imaging Multi-Mode reader (Cytation 1, BioTek) and was used for normalizing/correcting OCR results. Data were collected using Wave 2.6.1 Desktop software (Agilent Technologies) and exported to Prism 9 (GraphPad) for further analysis according to the manufacturer’s instructions.
The OCR of intact muscle tissue was measured as previously described93,158. In brief, mice were starved for desired durations and were killed through cervical dislocation. The gastrocnemius muscle from two hindlegs were then excised, followed by incubating in 4 ml of dissociation medium (DM; by dissolving 50 μg ml–1 gentamycin, 2% (v/v) FBS, 4 mg ml–1 collagenase A in DMEM containing HEPES) in a 35-mm culture dish in a humidified chamber at 37 °C, 5% CO2, for 1.5 h. The digested muscle mass samples were then washed with 4 ml pre-warmed collagenase-A-free DM, incubated in 0.5 ml pre-warmed collagenase-A-free DM and dispersed by passing through a 20 G needle 6 times. Next, 20 μl of muscle homogenate was transferred to a well of a Seahorse XF24 Islet Capture microplate (Agilent Technologies). After placing an islet capture screen by a Seahorse Capture Screen Insert Tool (Agilent Technologies) into the well, 480 μl pre-warmed aCSF medium (120 mM NaCl, 3.5 mM KCl, 1.3 mM CaCl2, 0.4 mM KH2PO4, 1 mM MgCl2, 5 mM HEPES, 15 mM glucose, 1× MEM non-essential amino acids, 1 mM sodium pyruvate and 1 mM GlutaMAX; adjusted to pH 7.4 before use) was added, followed by equilibrating in a CO2-free incubator at 37 °C for 1 h. OCR was then measured at 37 °C in a XFe24 Extracellular Flux analyzer (Agilent Technologies), with a Seahorse XFe24 sensor cartridge (Agilent Technologies) pre-equilibrated in Seahorse XF Calibrant solution (Agilent Technologies) in a CO2-free incubator at 37 °C overnight. Respiratory chain inhibitors used during the assay were oligomycin at 100 μM, FCCP at 100 μM, 50 μM antimycin A and 1 μM rotenone (all final concentrations). Data were collected using Wave 2.6.3 Desktop software (Agilent Technologies) and exported to Prism 9 (GraphPad) for further analysis according to the manufacturer’s instructions.
Determination of the critical micelle concentration of LCA
The critical micelle concentration (CMC) of LCA was determined using a fluorometric assay, with some modifications159,160. In brief, 1 μl Nile Red dye (5 mM stock solution dissolved in DMSO) was diluted with 2 ml PBS or DMEM (without phenol and supplemented with sodium pyruvate and GlutaMAX) in a quartz cuvette. The LCA stock solution at a concentration of 100 μM (prepared by diluting 100 mM LCA solution in DMSO with PBS or DMEM in a 1:1,000 ratio) was then gradually added to the Nile Red mixture, with 20 μl added each time to create a series of concentrations ranging from 0 to 12 μM (at concentrations higher than 12 μM, LCA precipitated in the medium). As a control, equal concentrations of Tween-20 were added to the mixture. The fluorescence of Nile Red was excited at 543 nm and recorded with a PMT detector at a voltage of 600 V, covering the emission spectrum of Nile Red (570–700 nm) by a Cary Eclipse spectrophotometer (Varian). The emission intensities recorded at each concentration of LCA were plotted to the concentration of LCA, and the inflection point of a Boltzmann fit of the resulting sigmoidal curve provides the CMC of LCA.
Statistical analysis
Statistical analyses were performed using Prism 9 (GraphPad Software), except for the survival curves, which were analysed using SPSS 27.0 (IBM) by log-rank (Mantel–Cox) test. Each group of data was subjected to Kolmogorov–Smirnov tests, Anderson–Darling tests, D’Agostino–Pearson omnibus tests or Shapiro–Wilk tests for normal distribution when applicable. An unpaired two-sided Student’s t-test was used to determine the significance between two groups of normally distributed data. Welch’s correction was used for groups with unequal variances. An unpaired two-sided Mann–Whitney test was used to determine the significance between data without a normal distribution. For comparison between multiple groups with two fixed factors, an ordinary two-way ANOVA or two-way repeated-measures ANOVA (for blood glucose measured during GTT, ITT and clamping) was used, followed by Tukey’s or Sidak’s multiple comparisons test as specified in the figure legends. The assumptions of homogeneity of error variances were tested using F-test (P > 0.05). Geisser–Greenhouse’s correction was used where applicable. The adjusted means and s.e.m., or s.d., were recorded when the analysis met the above standards. Differences were considered significant when P < 0.05, or P > 0.05 with large differences of observed effects (as suggested in refs. 161,162).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.