Animal procedures
C57BL/6J (Jax stock: 000664), Ai75 (Jax stock: 025106), Ai14 (Jax stock: 007914), Sun1-sfGFP (Jax stock: 030952)51, vGAT-Cre (Jax stock: 028862), PV-Cre (Jax stock: 008069), vGluT2-Cre (Jax stock: 028863), SST-Cre (Jax stock: 013044) and CAMKII-Cre (Jax stock: 005359) mice were purchased from The Jackson Laboratory and bred in house. Genotyping for each line was performed using primers recommended by The Jackson Laboratory (https://www.jax.org/). TRAP2 mice31 (a gift from L. Luo’s laboratory, also Jax stock: 030323) were crossed for specific experiments with Ai75 or C57BL/6J mice. Only male mice were used for experiments, and all mouse lines were maintained on a C57BL/6J background. Heterozygotes for Fos2A-iCreER and Ai75 alleles were used in behaviour tests. Mice were ordered from The Jackson Laboratory, and acclimated at the Stanford animal facility for at least two weeks. Mice were fed ad libitum on the ENVIGO (T2918.15) diet throughout the study. Mice were housed in groups with up to five mice per cage and on 12-h light–dark cycles (07:00–19:00, light) before behaviour experiments. After STFP training, test mice were single-housed until food-choice tests were done. All behaviour experiments were performed during the same circadian period by experimenters unaware of the subject identity. All protocols and husbandry conditions were approved by the Administrative Panel on Laboratory Animal Care at Stanford University under the guidelines of the National Institutes of Health for the care and use of laboratory animals.
Pharmacological agents
Tamoxifen (Sigma, T5648) stock solutions were prepared by dissolving tamoxifen in corn oil (Sigma, C8267) in the presence of ethanol, which was then evaporated before use in a speed vac as described52. Tamoxifen was administered intraperitoneally once daily at 150 mg per kg from day 1 to day 5 after STFP training. CNO (Tocris, 4936) or saline vehicle was administered intraperitoneally at 2 mg per kg twice daily or 40 min before the food-choice test. For experiments in which CNO was injected during the entire three weeks or only during the third week, CNO injections were stopped 24–48 h before the three-week food-choice test. For the CNO terminal infusion into the AONm, 200 nl CNO at 2.5 pg nl−1 was delivered bilaterally through an infuser connected to a microinfusion pump (WPI, SP101I), which was left in place for an additional 2 min to allow the drug to fully diffuse before extraction. CNO or vehicle saline was microinfused twice daily from day 1 to day 7 after STFP training for the experiments in Fig. 6b. Anisomycin (Sigma A9789) was prepared as described53 and infused into the OFC through an infuser or stereotactically injected into the COApm immediately after STFP training.
Plasmid construction and AAV preparations
AAV-DO_DIO (Addgene 37120), TeNT, non-floxed SynaptoTag and Cre-on SynaptoTag constructs were described previously33,54,55. For Cre-off SynaptoTag and Cre-off TeNT, the elements between the two loxPs were flipped55. For HA-Cre, the GFP moiety of Cre-GFP was replaced with an HA tag. Plasmids were converted into adeno-associated viruses (AAVs) with the AAV-DJ56, AAV2retro57 or AAV1(AAV1-Cre)58 serotype. In brief, helper plasmid (phelper) and capsid plasmids (pDJ or AAV2retro) were co-transfected with virus plasmid into HEK293T cells (ATCC, CRL-11268) using calcium phosphate. Then, 72 h after transfection, cells were collected and lysed, and the supernatant was loaded onto an iodixanol gradient medium (Accurate, AN1114542) and ultracentrifuged at 65,000 rpm at 4 °C for 3 h. AAVs were then extracted from the 40% iodixanol layer, washed, concentrated, aliquoted and stored at −80 °C until use. hSyn-DIO-hM4Di-IRES-GFP AAVs were a gift from X. Chen’s laboratory at Stanford University.
Stereotactic injections and cannula implantation
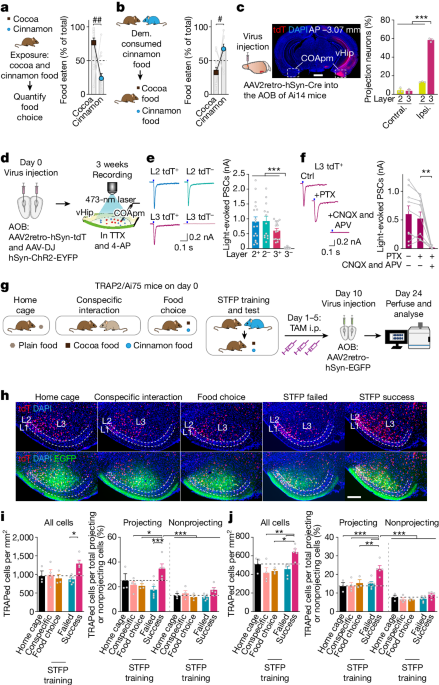
Eight-week-old mice were anaesthetized with 250 mg per kg tribromoethanol (Sigma, T48402). Carprofen (5 mg per kg) was injected subcutaneously before and after surgery as an anti-analgesic. The following coordinates were used (AP, anterior to bregma; ML, lateral to midline; DV, ventral to dura; in mm): (1) COApm, AP −2.80, ML ±2.85, DV −5.1; (2) AONm, AP +2.33, ML ±0.5, DV −3.0; (3) mPFC, AP +2.0, ML ±0.3, DV −2.0; (4) OFC, AP +2.7, ML ±1.2, DV −1.8; (5) BLA, AP −1.4, ML ±3.4, DV −4.5; (6) ventral hippocampus, two sets of coordinates were used, AP −3.3, ML ±3.2, DV −3.2 and −2.0; AP −3.3, ML ±2.5, DV −3.6 and −1.8. For the AOB, AP was recognized by both the distance from bregma +4.0 mm and posterior to the inferior cerebral vein, ML ± 0.88, DV −0.88. Before injecting the AOB, the skull was adjusted at an angle of around 30°, which made the bregma higher than the lambda, and surgeries were carefully conducted to avoid damaging the inferior cerebral vein. Viruses were injected through a beveled glass pipette connected to a nanolitre injector (WPI, NL2010MC2T) at a rate of 0.1–0.25 μl per min. Injection started 1 min after the glass pipette had reached the DV depth, and the glass pipette was removed slowly 10 min after the injection was done.
For AONm drug infusions, the bilateral guide cannula (2.1 mm in length, 1.2 mm centre to centre) was implanted above the AONm and used with an infuser (33 gauge, 1.0 mm projection). For AOB drug infusions, the bilateral guide cannula (0.88 mm in length, 2 mm centre to centre) was implanted above the AOB and used again with an infuser (0.5 mm projection). For OFC infusions, the bilateral guide cannula (1.3 mm in length, 2.2 mm centre to centre) was implanted above the OFC and also used with an infuser (0.5 mm projection). Because the implantation of a cannula above the COApm will damage the ventral hippocampus that is also essential for the STFP memory acquisition, we stereotactically injected anisomycin into the COApm immediately after STFP training.
Biocytin labelling to map local dendrites of neurons was performed by patching neurons and filling them with biocytin, followed by imaging. Neurons were identified after labelling them with two approaches, infection of the AOB of C57BL/6J mice with a mixture of AAV expressing anterograde EYFP and AAV2retro-hSyn-tdTomato viruses, or infection of Sun1-sfGFP mice with a mixture of AAV expressing anterograde mCherry and AAV2retro-hSyn-Cre-HA viruses.
The intervals between virus injections and analyses are stated in the figures, except for the SynaptoTag tracing experiments, in which eight-week-old C57/BL6J mice were injected with viruses and analysed six to eight weeks afterwards.
Behavioural tests
Production of flavoured food pellets and innate food-preference tests
The production of flavoured food pellets and the innate food-preference tests were performed as described7,13. In brief, scented food pellets were made using food powders produced in a blender from normal mouse chow (ENVIGO, T2918.15). Food powders were mixed with ground cinnamon (McCormick; final concentration of 1%), cocoa powder (Hershey’s, 100%, non-sweetened; final concentration of 2%59), ground cumin (McCormick; final concentration of 0.5%) or ground thyme (McCormick; final concentration of 0.75%38). For innate food-preference tests, mice naive to the odours used were fasted for 15–18 h and then given two food choices (cocoa versus cinnamon, or cumin versus thyme) for one hour. The food pellet was weighed before and after the test. The proportion of each flavoured food consumed was calculated as the ratio to the total food consumed. In all figures, cinnamon-flavoured food is represented by a solid circle, cocoa-flavoured food by a solid square, cumin-flavoured food by a hollow circle and thyme-flavoured food by a hollow square.
STFP training and tests
STFP was performed as illustrated in the schematic of Fig. 1a,b, with habituation, training and food-choice test sessions as described7,14,39,59,60,61,62,63,64, using two odour pairs (cinnamon versus cocoa2,13,59 or cumin versus thyme38). During habituation, both demonstrator (blue cartoon for cinnamon and yellow green for cumin) and observer mice (subject, brown cartoon) were singly housed in new cages with food deprivation for 12–15 h. Before STFP training, demonstrator mice were fed 1% cinnamon-flavoured or 0.5% cumin-flavoured food pellets for one hour. Only demonstrators that consumed more than 0.2 g food were used in subsequent STFP training sessions. Afterwards (during STFP training), demonstrator mice were allowed to socially interact with observer mice for 30 min in the absence of food. Food-choice tests (STFP memory tests) were performed immediately after STFP training and/or later as described in the figures with the observer mice that had been continuously single-housed and had been food-deprived for 12–15 h before the tests. In the food-choice tests, mice were offered cinnamon- and cocoa-flavoured or cumin- and thyme-flavoured food pellets for one hour. The food pellets were weighed before and after food-choice tests and the percentage of flavoured food eaten by observer mice was calculated. In all figures, data from three-week food-choice tests were shaded in grey to differentiate from the day 0 food-choice test data. The memory retention index was calculated as the ratio of the per cent cued food eaten in the 24-h, 48-h or 3-week food-choice test to the cued food eaten in the day 0 test. For the behaviour conducted in Extended Data Fig. 1b, observer mice were directly exposed to 1% cinnamon odour for 10 min. For Extended Data Fig. 1c, observer mice socially interacted with a toy demonstrator scented with 1% cinnamon food powder instead of a real demonstrator.
Note that in standard experiments (except where noted otherwise), observer mice were subjected to a food-choice test immediately after the training session (day 0 test). Observer mice were considered to be successfully STFP trained when the consumed cued food percentage exceeded 50%, and mice that did not learn the food odour as documented in the day 0 test were excluded from further analyses (except for the experiments in which memory acquisition was examined (Fig. 2a,e) or no day 0 test was performed (Extended Data Fig. 2c)). The success rate of STFP training was 70–90% for the cocoa and cinnamon odour pair, and around 50% for the cumin and thyme odour pair38 (Extended Data Fig. 2r). After the day 0 test, observer mice underwent only one additional food-choice test, at 24 h, 48 h or 3 weeks (‘STFP test’ in all schematics), with the following exceptions: for the experiments in Extended Data Fig. 1b,c,e, multiple food-choice tests were performed, whereas for the experiments in Extended Data Figs. 1d and 2c, no day 0 test was performed because these experiments aimed to ensure that the day 0 test did not influence long-term memory formation. In experiments using conspecific interaction controls or uncued food controls, demonstrator mice were fed with unscented food pellets but the procedure was otherwise the same.
Social behaviour during STFP training
Social behaviour during STFP training was recorded and analysed as described2,36,65. Observers’ sniffs of the demonstrator’s muzzle, body and anogenital region, as well as self-grooming bouts and fighting bouts, were annotated on a frame-by-frame basis using a MATLAB code BehaviorAnnotator (https://github.com/pdollar/toolbox). Pearson correlation analyses were performed between the behaviours scored and the percentage of consumed cued food15,66.
Buried food test
After food deprivation for 15–18 h, a test mouse was put into the centre of a new cage. A single normal food pellet was buried 1 cm under the bedding in a random corner. The latency the test mouse took to find the food pellet was video-recorded and measured offline13. The assay was finished within 5 min, so the latency for a test mouse that failed to dig up the pellet was recorded as 300 s.
Olfactory preference test
A 2 × 2-cm filter paper scented with water, 2-phenylethanol (10%, v/v) or 2-methylbutyric acid (10%, v/v)67 was sequentially provided to a test mouse after habituation. Each scented filter paper was placed in the cage at the opposite side of the test mouse for 3 min. The mouse behaviour was video-recorded and the total investigation time of the filter paper was scored blindly. The water-scented filter paper result was subtracted as the baseline from the total investigation time for the other two odours68.
Olfactory sensitivity test
A 2 × 2-cm filter paper containing a series of dilutions of isoamyl acetate in water (0, 0.001%, 0.01% and 0.1%) was placed in the opposite corner of the test mouse in a cage after cage habituation for 3 min. The sniffing time of each test mouse as a measurement of exploratory behaviour was video-recorded and quantified offline68.
Mapping odour-sensitive neurons using FOS staining
B6 mice were exposed to water, 2-phenylethanol or 2-methylbutyric acid applied to a 2 ×2-cm filter paper for 3 min, and then returned to their home cage. Ninety minutes after odour exposure, mice were anaesthetized and perfused. Brain slices from the mice were immunostained for FOS.
Non-associative olfactory memory
Non-associative olfactory memory was analysed as described13. In brief, cinnamon extract or anise extract was mixed with distilled water to a final concentration of 1%. On day 1, mice were allowed to freely sniff the odours in the chamber of the open field test used above for 10 min as an initial preference test. On day 2, mice were first exposed to the cinnamon odour in their home cage for 15 min. Then, after 30 min, the mice were returned to the chamber with the anise and cinnamon odours in two different random corners for another 10 min of sniffing. The anise preference index was calculated by dividing the investigation time of anise by that of cinnamon. The non-associative memory index was calculated by dividing the anise preference index of the second day (pre-exposure) by that of the first day (naive). Behaviour was recorded using the Viewer III tracking system, and analysed on a frame-by-frame basis using a MATLAB code BehaviorAnnotator (https://github.com/pdollar/toolbox) according to the previous description.
Fear conditioning
Fear conditioning experiments were conducted to evaluate contextual memory33,54. On day 1, the test mouse was trained in the fear conditioning chamber by pairing a 30-s, 80-dB, 2-kHz tone with a 2-s, 0.75-mA foot shock. On day 2, contextual memory was tested by placing the mice back into the fear conditioning chamber for 5 min. On day 3, altered context and tone tests were performed in a modified chamber in which the walls and the chamber bottom were covered with plastic sheets with colourful paintings or stripes. The test mouse was placed in the modified chamber for 5 min to measure altered context memory, followed by 1 min of tone (80 dB, 2 kHz) to measure the tone-associated memory. All behaviour was video-recorded and ‘freezing’ was quantified using FreezeView software (Coulbourn Instruments).
Open field tests
Open field tests were performed by placing a test mouse in the centre of a 40 × 40 × 40-cm open field box. The test mouse was given 15 min for free exploration. Behaviour was video-recorded and analysed using a BIOBSERVE III tracking system. The centre zone was defined as the central 20 × 20-cm square of the box centre manually during analysis, and the total distance travelled and time spent exploring the centre area were measured.
Three-chamber social behaviour
Three-chamber social behaviour was performed as described33. In brief, control and test mice expressing DREADDs or GFP were intraperitoneally injected with CNO 40 min before the test. Mice were placed at first in the central chamber to freely investigate all three chambers for 10 min. During the subsequent sociability test, a sex- and age-matched stranger mouse (stranger 1) was placed inside an upside-down wire pencil cup in one of the side chambers and an empty cup in the other side, and the exploratory behaviour of the test mouse was video-recorded for 10 min. During the following social novelty test, a second stranger mouse (stranger 2) was placed into the empty pencil cup of the three-chamber set-up and the exploratory behaviour of the test mouse was again video-recorded for another 10 min. The time mice spent in each chamber was analysed using the BIOBSERVE III tracking system.
Slice electrophysiology
Slicing
Mice were anaesthetized using isoflurane, and brains were quickly removed into an ice-cold sucrose-based cutting solution (in mM: 228 sucrose, 26 NaHCO3, 11 glucose, 2.5 KCl, 1 NaH2PO4, 0.5 CaCl2 and 7 MgSO4, oxygenated by 95% O2 and 5% CO2). Coronal brain slices (300 μm) containing the COApm were sectioned with a vibratome (VT1200S, Leica Biosystems) and recovered in oxygenated artificial cerebrospinal fluid (ACSF, in mM: 119 NaCl, 26 NaHCO3, 2.5 KCl, 10 glucose, 1 NaH2PO4, 2.5 CaCl2 and 1.3 MgSO4) first at 32 °C for 30 min and then at room temperature for another 1 h. Slices were afterwards transferred to an electrophysiological recording chamber in which they were perfused with ACSF at 1 ml per min at 32 °C.
Optogenetic recordings
For verification of monosynaptic connections between AOB inputs and COApm neurons, 1 μM TTX and 100 μM 4-AP were included in the ACSF, and recordings were done as described33. The COApm was visualized with an upright microscope (Olympus, BX51WI) under a 60× water immersion objective. A 473-nm blue laser light was delivered to the COApm for 1 ms through a customized digital micromirror device-based photostimulation optogenetic system33. Layer 2 and layer 3 were distinguished from the distance to layer 1 and the intensity of neurons. Layer-2 tdT+, layer-2 tdT−, layer-3 tdT+ and layer-3 tdT− neurons were all recorded through whole-cell voltage-clamp recordings. Glass pipettes (2–3 MΩ) were filled with internal solutions (in mM): 135 CsCl, 1 EGTA, 10 HEPES, 4 ATP-Mg, 0.1 spermine, 0.3 GTP-Na and 7 phosphocreatine (pH 7.2–7.30, osmolarity adjusted to 300–310). Picrotoxin (PTX; 50 μM), 50 μM APV and 20 μM CNQX were sequentially added in ACSF to determine whether light-evoked postsynaptic currents were inhibitory or excitatory. Neurons were clamped at −70 mV during recordings.
Optogenetic analyses of AMPAR-mediated synaptic plasticity
For optogenetic analyses of AMPAR-mediated synaptic plasticity, the following internal solution was used (in mM): 135 CsMeSO3, 1 EGTA, 10 HEPES, 4 ATP-Mg, 0.3 GTP-Na, 0.1 spermine and 7 phosphocreatine (pH 7.2–7.30, osmolarity adjusted to 300–310). In the AMPAR/NMDAR ratio experiment, 1 μM TTX, 100 μM 4-AP and 50 μM PTX were added in ACSF. Cells were held at −90 mV and given a 1-ms blue-light photostimulation to record AMPAR responses and then switched to +40 mV to record NMDAR responses. The peak of NMDAR-dependent light-evoked responses was measured at 50 ms after the onset of currents. The AMPAR/NMDAR ratio was calculated as NMDAR currents divided by the AMPAR currents. In AMPAR rectification experiments, 50 μM PTX and 50 μM APV were included in the ACSF with 1 μM TTX and 100 μM 4-AP. Blue-light-evoked AMPAR currents were recorded at −70 mV, 0 mV and +40 mV, respectively. The rectification index was calculated by absolute values of AMPAR currents at −70 mV divided by AMPAR currents at +40 mV.
Intrinsic excitability recordings
For intrinsic excitability recordings, whole-cell current-clamp recordings were achieved in layer-3 tdT+ neurons using the following internal solution (in mM): 135 K-gluconate, 10 HEPES, 0.25 EGTA, 1 MgCl2, 4 ATP-Mg, 0.3 GTP-Na, 0.1 spermine and 7 phosphocreatine (pH 7.2–7.30, osmolarity adjusted to 300–310). PTX (50 μM), 20 μM CNQX and 50 μM APV were included in the ACSF to block synaptic transmission69. After whole-cell recordings were established under voltage clamp, cells were switched to current clamp. Depolarizing currents from 0 pA to 250 pA (stepped by 50 pA, 1 s) were injected, and action potentials were recorded under current clamp. The current–frequency relationship was fitted with a single exponential equation70 in a transformed version: frequency = a × log10(current injections) − a × log10(I0), where I0 is the minimal current to elicit spikes. We calculated input resistances using Ohm’s law. Specifically, we injected currents ranging from −200 pA to +50 pA in 50-pA steps into neurons under current clamp and recorded the resulting voltage changes. The slope of the current–voltage relationship was then calculated as the input resistance. Resting membrane potentials were monitored after the stable establishment of whole-cell recordings without current injections. Action potential properties were analysed using parameters previously reported30.
Mini event recordings
To verify TeNT efficiency, mice were euthanized one week after virus injection and miniature EPSCs (mEPSCs) were monitored for 5 min in acute COApm brain slices in the presence of 1 μM TTX and 50 μM picrotoxin.
All junction potentials were not corrected. Cells were rejected for further analysis if series resistances changed more than 20% during recordings. All electrophysiological data were recorded using the MultiClamp 700B amplifier, digitalized at 10 kHz with Digidata1440, with Clampex 10.4, and analysed with Clampfit 10.4 (Molecular Devices).
Biocytin labelling
During whole-cell recordings, biocytin (2 mg ml−1, Sigma, B4261) was included in CsCl-based internal solutions71. After recordings, recording electrodes were removed slowly and slices were immediately fixed in ice-cold 4% paraformaldehyde (PFA)/phosphate-buffered saline (PBS) solutions. Slices were washed in PBS for 5 min three times and then permeabilized and blocked in blocking buffer (containing 5% goat serum + 0.3% Triton X-100 in PBS) at room temperature for 1 h. Then Streptavidin Fluor 647 conjugate (S21374, Invitrogen, 1:1,000) was added for 2 h incubation at room temperature. Slices were then washed in PBS for 15 min, repeated four times, and were moved to PBS with DAPI (Sigma, D8417) to stain for another 15 min. After staining was done, slices were mounted onto Superfrost Plus slides with mounting medium (Fluoromount-G, 0100-01, SouthernBiotech). Images were taken with a Nikon confocal microscope (A1Rsi, Nikon, Japan) equipped with a 60× oil objective.
Immunohistochemistry
Mice were deeply anaesthetized with isoflurane and transcardially perfused by PBS followed by ice-cold 4% PFA in PBS. For staining with anti-glutamate and anti-GABA antibodies, brains were placed into 30% sucrose/PBS solutions for cryoprotection without post-fixations. Otherwise, brains were post-fixed in 4% PFA overnight and switched into 30% sucrose/PBS solutions for another two days before further processing. Coronal COApm sections and sagittal AOB sections (both 40 μm thickness) were cut with a Lecia CM3050-S cryostat and incubated first at room temperature for 1 h in a blocking solution (5% goat serum and 0.3% Triton X-100 in PBS) and then at 4 °C overnight with primary antibodies (anti-glutamate, rabbit polyclonal, 1:1,000, Sigma-Aldrich G6642; anti-GABA, rabbit polyclonal, 1:1,000, Sigma-Aldrich A2052; anti-NeuN, mouse monoclonal, 1:1,000, Millipore, MAB377; anti-GFP, rabbit polyclonal, 1:1,000, Invitrogen A11122; anti-mCherry, rat monoclonal, 1:1,000, Invitrogen M11217; anti-FOS, Synaptic System 226308, guinea pig, 1:1,000). After 3× 15 min washing in PBS, sections were incubated with fluorescent secondary antibodies (goat anti-rabbit Alexa Fluor 488, Thermo Fisher Scientific, A11034; goat anti-rat Alexa Fluor 546, Thermo Fisher Scientific, A11081; goat anti-mouse Alexa Fluor 647, Thermo Fisher Scientific, A21236; for biocytin labelling, Streptavidin Fluor 647 conjugate, S21374, Invitrogen) in blocking buffer for 2 h at room temperature, washed 4× 15 min in PBS stained for 15 min with DAPI (Sigma, D8417) and mounted onto Superfrost Plus slides with mounting medium (Fluoromount-G, 0100-01, SouthernBiotech) for imaging.
SynaptoTag tracing of efferent synaptic connections from the AOB
Three SynaptoTag constructs (non-floxed SynpatoTag, Cre-on SynaptoTag, and Cre-off SynaptoTag) were used. AAVs of these constructs were injected into the COApm either without or with prior injection of retro-AAVs encoding Cre recombinase into the AOB of six-to-eight-week-old wild-type C57BL/6J mice. Whole-brain coronal sections (40 μm) were collected from the beginning of the olfactory bulb to the end of the cerebellum six to eight weeks after injections. Every fifth section was stained with anti-GFP and anti-mCherry, mounted onto the Superfrost Plus slides in a rostral to caudal sequence and imaged using a Slide scanner (Olympus, VS200 or BX61VS) with a 10× objective. Mice were included in the analysis only when the virus injection accurately targeted the COApm.
Retrograde trans-synaptic pseudotyped rabies virus tracing
Cell-specific monosynaptic rabies tracing was performed as described50,72. A 1:1 volume mixture of AAV5-CAG-DIO-TVA-mCherry (avian tumour virus receptor A) and AAV8-CAG-DIO-G (glycoprotein) was injected into the COApm (0.2 μl in total) unilaterally, whereas AAV2retro HA-tagged Cre was injected into the AOB of the same hemisphere of eight-week-old C57BL/6J mice. Two weeks after AAV injections, 0.2 μl of RVdG (GFP-tagged G-deleted rabies virus) was injected into the same COApm. Six days after RVdG injection, mice were perfused and fixed with PFA and their brains were analysed as described for the SynaptoTag mapping, using immunohistochemistry for GFP to detect input cells. All viruses used in rabies tracing were produced by the Janelia Farm Viral Core Facility.
Imaging and image quantifications
Slides from the same experiments were imaged in parallel with the same settings using an Olympus Slide scanner. Quantifications of rabies and SynaptoTag tracings were performed as described73 with modifications. Brain regions were recognized under DAPI with the help of NeuroInfo Software (MBF Bioscience) under the guidance of the Franklin and Paxinos mouse brain atlas74 and the Allen Reference Atlas (https://atlas.brain-map.org/). For Fig. 1c and Extended Data Fig. 1i,j, the percentage of neurons was quantified by NeuN staining. For retrograde pseudotyped rabies virus tracings, cell bodies were counted manually with a cell counter. Input brain regions were presented as the percentage of GFP-positive cells among the total GFP-positive cells in the whole brain. For quantifications of presynaptic terminals using SynaptoTag (Syb2GFP), the averaged intensity of GFP signals of each brain region was measured by ImageJ and the background of each section was subtracted. To correct the variations caused by the different levels of virus injections and expression, the intensity of every brain region was normalized to the intensity of the injection site in each mouse’s COApm, which was identified by soma-expressed mCherry signals. For TRAP2 mapping, the cell layers of the COApm were delineated through the DAPI signal and the background of fluorescent channels. Cells labelled by tdTomato, GFP or both tdTomato and GFP, or DAPI only, were counted using the cell counter in ImageJ or CellProfiler. The percentage of activated cells among projection neurons was calculated as tdT+ and GFP+/total GFP+ cells × 100, and the percentage of activated cells in nonprojection neurons was calculated as tdT+ and GFP−/(DAPI-labelled cell nuclei – GFP+ cells) × 100.
scRNA-seq and data analyses
Single-cell dissociation and flow cytometry (FACS)
AAV2retro-hSyn-tdTomato viruses were bilaterally injected into the AOB two weeks before the experiments. On the experiment day, mice were treated as follows: (1) mice in the ‘odour group’ were exposed to 1% cinnamon odour on a filter paper and then given the cocoa and cinnamon food choice; (2) mice in the STFP group were subjected to general STFP protocols (see above); that is, were enabled to socially interact with demonstrator mice who consumed 1% cinnamon-flavoured food and were then given the cocoa and cinnamon food choice; (3) mice in the home-cage group were not subjected to odour or STFP treatment, but otherwise were processed in parallel with the other two groups, and given normal food chow instead of the cocoa- and cinnamon-flavoured food pellets. All mice were single-housed and fasted. Thirty minutes after treatments, mice were euthanized and single neurons from the COApm were dissociated and sorted by fluorescence-activated cell sorting (FACS) as described48. In brief, mice were anaesthetized with isoflurane and decapitated quickly. Brains were removed into ice-cold choline chloride-based ACSF (in mM: 110 choline chloride, 24 NaHCO3, 20 glucose, 1.3 NaH2PO4, 2.5 KCl, 0.5 CaCl2, 7 MgCl2, 3 sodium-pyruvate,1.3 sodium-ascorbate, 2 thiourea and 13.2 trehalose, oxygenated by 95% O2 and 5% CO2). Coronal brain slices (300 μm) were cut using a vibratome (VT1200S, Leica Biosystems). Brain slices containing COApm were collected, and COApm was dissected under a fluorescence dissection microscope as accurately as possible according to the boundaries of the COApm, guided by retrogradely expressed tdTomato. Microdissected COApm tissues were incubated at 34 °C in papain enzyme mix containing DNase (LK003150, Worthington) with 800 nM kynurenic acid for 20 min. The tissue was gently triturated with a P1000 pipette, repeated every 15 min three times or until fully dissociated. After dissociation, cell suspensions were centrifuged at 350g for 10 min at room temperature. The supernatant was discarded and cell pellets were carefully resuspended in 1 ml oxygenated EBSS (with 10% ovomucoid inhibitor, 4.5% DNase and 800 nM kynurenic acid) and centrifuged, and the cell pellets were washed with 1 ml ACSF including 0.1% RNAse inhibitor. A 70-μm cell strainer (Thermo Fisher Scientific, 352350) was used to remove debris. Cells were stained with Hoechst (1:2,000; H3570, Life Technologies) for 10 min, washed and resuspended in ACSF. Cells were kept on ice or at 4 °C before they were sorted by FACS using a Sony SH800 sorter directly into 384-well plates with lysis buffer containing oligodT. Singlets were selected on the basis of Hoechst signals, and all Hoechst-positive singlet cells were collected48 (see Supplementary Fig. 1 for sorting strategy). Cells were sorted at a low rate, but each plate was done within 25 min. After FACS, plates were sealed, centrifuged and immediately snap-frozen and stored at −80 °C until further processing.
Library preparation and sequencing
The library was prepared according to the Smart-seq-2 protocol in a 384-well format75. In brief, cDNA was amplified by 23 PCR cycles. A PicoGreen quantitation assay in the 384-well format was used to assess cDNA concentrations, which were normalized to around 0.4 ng µl−1 per sample automatically performed by the TPPLabtech Mosquito HTS and Mantis (Formulatrix) robotic platforms. An in-house Tn5 was used to prepare, pool and clean libraries. Libraries were then sequenced on a Novaseq instrument (Illumina) using 2× 100-bp paired-end reads and 2× 12-bp index reads with a 200-cycle kit. Averaged sample reads per cell were 1.5 million.
Bioinformatics and data analysis
First, sequences obtained from Novaseq were de-multiplexed using bcl2fastq. Next, reads were aligned to the mouse mm10 genome (with tdTomato sequences added) augmented with ERCC (External RNA Controls Consortium) sequences using STAR (v.2.7.10a)76. We determined gene counts using FeatureCounts (v.2.0.0)77. We used standard algorithms and procedures for cell filtering, feature selection, dimensionality reduction and clustering. Genes were removed if they appeared in fewer than five cells. Cells with fewer than 500 genes or with fewer than 150,000 reads were also removed. In addition, cells with more than 5% reads as ERCC, and more than 5% mitochondrial transcripts, were also excluded from analysis. We log-normalized counts for each cell and scaled using ScaleData if necessary and appropriate48. This resulted in a dataset of 3,315 total cells, including 1,694 neurons.
Cells were visualized using UMAP. First, we aligned the raw data from all groups using the first ten canonical components of the ‘canonical correlation analysis’ function from the Seurat package (v.4.9.9)78. Principal component analysis was performed on projected genes into the principal component space. Single-cell principal component scores and gene loadings for the first 30 principal components were computed. Seurat’s FindClusters and Runumap functions were then used to calculate two-dimensional UMAP coordinates78.
We performed DEG analysis in three dimensions by applying the Mann–Whitney U-test to various cell populations. We used a P < 0.01 and log2-transformed fold change (log2FC) > 1 in both the STFP versus odour and the STFP versus home-cage comparisons. First, we identified DEGs between tdT− and tdT+ cells within neuron cluster 1, separately in the three groups. Second, we analysed DEGs of neuron clusters between groups—namely, odour versus home cage, STFP versus home cage and STFP versus odour. Next, we identified exclusive DEGs by removing DEGs that are also present in tdT− neurons as well as the odour and home-cage conditions. In detail, we identified DEGs in comparisons of AOB-projecting (tdT+) neurons in the STFP and the odour-only conditions. We then removed DEGs that are also differentially expressed in non-AOB-projecting neurons, allowing the identification of changes that were specific to AOB-projecting neurons that are selectively essential for long-term STFP memory. We also removed DEGs that were differentially expressed between the odour-only and the home-cage conditions to ensure that DEGs were not a consequence of an odour experience. These criteria produced a set of ‘STFP-specific DEGs’ (Supplementary Table 4 and Fig. 5f). All raw P values were adjusted using Benjamini–Hochberg correction66. All graphs and analyses were generated and performed in R (v.4.2.2).
MERFISH spatially resolved transcriptomics
MERFISH experiments were performed as described79. The same behavioural design as was used in the scRNA-seq experiment was applied in the MERFISH experiment with three groups: (1) home-cage group; (2) odour group; and (3) STFP group.
MERFISH gene selection
To determine the optimal genes for MERFISH, we combined insights from the scRNA-seq data and the relevant literature. Our strategy centred on pinpointing marker genes for specific cell types using a comparative approach. (1) Identification process: we used the Mann–Whitney–Wilcoxon test to compare each gene’s expression between cells of a target population and all other cells. We then adjusted the resulting P values for multiple-hypothesis testing, yielding FDR-adjusted P values. (2) Selection criteria: the gene must be expressed in a minimum of 30% of cells in the target population. It should have an FDR-adjusted P value smaller than 0.001. Its expression in the target population should be at least four fold higher than the average in non-target cells. The proportion of cells expressing the gene in the target population should be at least twice as high as in any other cell group. Marker genes were ranked on the basis of their expression fold change compared with non-target cells. (3) We retained the top five marker genes from each cell type for further consideration. Beyond this data-driven approach, we also incorporated established genes linked to microglia, astrocytes and oligodendrocyte precursor cells (OPCs), as found in the literature. Furthermore, DEGs associated with remote memory were included, culminating in a comprehensive panel of 336 genes. Probes were designed using the Vizgen platform.
Tissue processing for MERFISH
Mice were anaesthetized and euthanized, and their brains were quickly dissected and frozen in OCT and stored at −80 °C until sectioning. Ten-micrometre-thick coronal sections containing the OFC or COApm and ventral hippocampus were collected using a Leica CM3050-S cryostat and directly mounted onto MERSCOPE slides for MERFISH analyses. Four coverslips of tissues were collected per mouse.
Sample preparation and MERFISH imaging
Slides were processed according to the MERSCOPE protocol (Vizgen). Slides were first washed three times in PBS, then permeabilized in 70% ethanol at 4 °C for 18 h. Slides containing tissue sections were then washed with sample preparation wash buffer (PN20300001) and incubated with formamide wash buffer (PN 20300002) for 30 min at 37 °C. Next, slides were incubated with the gene panel mix (RNA probes) at 37 °C for 48 h for hybridization and washed twice for 30 min in formamide wash buffer at 47 °C to remove excess coding and poly-A-anchor probes. The sections were then cleared by embedding in 4% polyacrylamide gel, followed by treatment with clearing premix (PN 20300003) at 37 °C for 36 h. Sections were then washed twice in sample preparation wash buffer, stained with DAPI/PolyT for an additional 15 min, washed with formamide wash buffer, again washed in sample preparation wash buffer and loaded into the MERSCOPE flow chamber. Images were captured at both 20× and 63× magnifications.
MERFISH data processing and analysis
MERFISH imaging data were processed using the MERlin pipeline80 with cell segmentation using CellPose81. Decoded molecules were registered and assigned to each cell as a MERFISH data matrix for further analysis. The MERFISH matrix for each section was concatenated, normalized, log-transformed with Scanpy82 and integrated using Harmony83. Leiden84 clustering was applied to generate cell clusters. DEGs identified in comparisons between groups or between tdTomato-positive and tdTomato-negative excitatory neurons were assessed using the Mann–Whitney–Wilcoxon test.
Statistics and reproducibility
All experiments and data analyses were performed on anonymized samples or anonymized animals, except for the viral tracing experiments, in which the experimental condition can be identified on the basis of the pattern of virus expression. For quantitative imaging experiments, the number of replicates of ‘representative images’ is the same as the number of replicates specified for the corresponding quantifications. For non-quantitative imaging procedures, the number of replicates is the same as in the corresponding analysis experiments, or, as for all experiments, the experiments were repeated at least three times. All images and numbers were checked for inadvertent duplications using duplication detection software, although in several instances the same numbers resulted in different experiments and were retained. Statistics tests were performed by Prism v.10 or SPSS v.28, or by R software (for the scRNA-seq and MERFISH analyses). We first checked all data, except for the scRNA-seq and MERFISH data, for normality distribution using the Shapiro–Wilk or Kolmogorov–Smirnov tests. Then, we checked the equality of variances of all data using the Brown–Forsythe test. Then, parametric or nonparametric tests were applied accordingly with post-hoc tests for multiple comparisons. When datasets passed normality and equal variances tests, parametric tests such as two-tailed paired or unpaired Student’s t-tests, one- or two-way ANOVA tests or repeated-measures ANOVA tests with Tukey post-hoc tests were applied. If these were failed, nonparametric tests, such as two-tailed unpaired Mann–Whitney or Wilcoxon matched-pairs signed-rank tests or Kruskal–Wallis tests with post-hoc two-stage linear step-up tests were applied, with the adjusted P value used to determine significance in the post-hoc two-stage linear step-up test. For two-way ANOVA tests, if the data were not normally distributed, they were first transformed to ensure that they were in a Gaussian distribution. For the two-tailed Student’s t-tests, effect size and 95% confidence interval were calculated related to the standard deviation. In Supplementary Table 5, Cohen’s d = (mean of group B − mean of group A)/pooled standard deviation. scRNA-seq and MERFISH data were processed and analysed in R.
All numerical data are expressed as means ± s.e.m. *P < 0.05, **P < 0.01 and ***P < 0.001 denote significance when comparing between groups or animals. #P < 0.05, ##P < 0.01 and ###P < 0.001 denote significance for within-animal comparisons. All statistics are indicated in the figure legends. Further details for statistics are provided in Supplementary Tables 5 and 6, including effect sizes and confidence intervals. All primary data are deposited in publicly accessible repositories (https://purl.stanford.edu/gy983cn1444).
Inclusion and ethics statement
As mandated by state and federal law, we were careful not to discriminate against anyone on the basis of race, ethnicity, gender, sexual orientation, age, ability, religion, socioeconomic status, nationality or any other protected or non-protected characteristic, and all individuals who contributed to the data collection are listed as co-authors regardless of race, ethnicity, gender, sexual orientation, age, ability, religion, socioeconomic status and nationality. Author contributions are listed in the ‘Author contributions’ section. Individuals who provided reagents, materials, suggestions and advice or any other types of contributions are acknowledged in the ‘Acknowledgments’ section.
This study was a collaboration between the laboratories of T.C.S. and S.R.Q. at Stanford University. The roles and responsibilities of the laboratories were discussed before the initiation of the experiments and were updated in step with discoveries emerging from the study. All required institutional approvals were obtained for this study from various committees at Stanford University, including full approval of the animal experiments from the Administrative Panel on Laboratory Animal Care at Stanford University. Requisite guidelines and regulations for research procedures, including for all animal experiments, were strictly followed.
As detailed in our laboratory’s publicly available manual, we are committed to an environment that values diversity, equity and inclusion on all perspectives, experiences and identities. We respect individuals from all backgrounds and are dedicated to promoting ethical conduct during our research. We do not tolerate any form of discrimination, harm, or disrespect in all aspects and uphold the dignity of all individuals. Moreover, we uphold all principles of intellectual honesty, plagiarism prevention and responsible citation practices that apply to research.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.