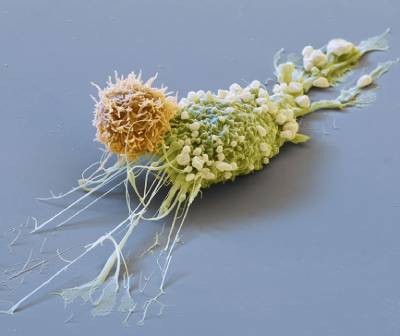
A cancer cell (blue; artificially coloured) is targeted by an engineered immune cell (purple), which can be enhanced by mutations originally discovered in cancer cells.Credit: Steve Gschmeissner/Science Photo Library
Cancer cells are the ultimate survivors, riddled with mutations that let them thrive when healthy cells would die. These same mutations can boost the ability of game-changing cell therapies to quash cancer, a study in mice shows1.
Among these therapies are chimeric antigen receptor (CAR) T cells, which are already used to treat several types of blood cancer. The new study shows that engineered CAR T cells carrying a mutation that was first found in cancerous T cells can vanquish tumours that don’t respond to current CAR-T therapies.
“It’s a beautiful piece of work and opens the door for better CAR-T therapies in the future,” says Madeleine Duvic, a dermatologist and cancer researcher at the MD Anderson Cancer Center in Houston, Texas, who was not involved in the work.
“Natural T-cell function isn’t good enough. We need to explore the extremes of T-cell function,” says Kole Roybal, an immunologist at the University of California, San Francisco, and co-author of the new paper. What better place to start than with the mutations that turn healthy T cells into hardier, cancerous ones?
The new approach was published today in Nature.
Cancer versus cancer
In the past few decades, scientists have developed bespoke cell therapies by harnessing the cancer-killing power of immune cells such as T cells. The most advanced of these treatments, CAR-T-cell therapies, rely on T cells collected from people with cancer. The cells are edited to express CAR proteins, which enable the T cells to seek and destroy cancer cells. The T cells are then re-infused into the person they came from.
Forget lung, breast or prostate cancer: why tumour naming needs to change
These living drugs have taken the research community by storm, and the US Food and Drug Administration has approved several CAR-T cell therapies for blood cancers such as lymphomas and multiple myeloma. But scientists are still struggling to work out whether these cells can be used to kill ‘solid’ cancers, such as breast and lung tumours.
Pulling from cancer’s playbook, Roybal and his colleagues incorporated 71 mutations, found in cancerous T cells, into CAR T cells. When they looked at how these perturbations affected T-cell function, one mutation stood out. The CAR T cells carrying an aberrant protein dubbed CARD11–PIK3R3 infiltrated well into tumours and had long-lasting cancer-killing activity.
“It’s a very special molecule, it seems to be able to beat all the tests we put to it,” says study co-author Jaehyuk Choi, a dermatologist at Northwestern University in Evanston, Illinois.
Potent cells
The team treated mice carrying blood and solid cancers with several T-cell therapies boosted with CARD11–PIK3R3, and watched the animals’ tumours melt away. Researchers typically use around one million cells to treat these mice, says Choi, but even 20,000 of the cancer-boosted T cells were enough to wipe out tumours.
Cancer-fighting CAR-T cells could be made inside body with viral injection
“That’s an impressively small number of cells,” says Nick Restifo, a cell-therapy researcher and chief scientist of the rejuvenation start-up company Marble Therapeutics in Boston, Massachusetts.
There is a risk that the supercharged cells will transform into cancers. But the animal data do not fuel any safety concerns, says Restifo, and the CARD11–PIK3R3 mutation seems to amp up edited T cells only when cancer cells are nearby, helping to mitigate worries about rogue immune cells.
Choi and Roybal have co-founded Moonlight Bio in Seattle, Washington, to move these cells towards use in people with cancer. They hope to have edited cells in clinical trials in two to three years. But the bigger opportunity is the chance to find other cancer mutations that will make T-cell therapies tick.
“A lot of people are going to think ‘Oh, this is such a good idea. Why didn’t I do this?’,” says Restifo.