Complex interactions between a mammalian host and its intestinal microbiota (the community of microorganisms that reside in the small and large intestines) influence host health and disease susceptibility. A major challenge in delving into the mechanistic relationships driving such interactions is the high diversity of species in the microbiota, which gives rise to a microbial profile that is unique to each individual1, much as a fingerprint is. There is a growing appreciation that the gut microbiota is implicated in generating resistance to gut colonization by disease-causing microorganisms (pathogens). However, many studies of this phenomenon have been largely descriptive, and have tended to correlate only particular microbiota compositions with a state of health or disease2. Writing in Cell, Stacy et al.3 present a detailed mechanism that reveals how changes to the microbiota drive resistance to invasion by pathogens.
It is generally accepted that the microbiota can hinder colonization by intestinal pathogens4, and several lines of evidence support the idea that the gut microbiota can have a role in limiting pathogen growth. For example, prolonged and/or high levels of antibiotic use in people promotes expansion of Clostridium difficile5, a bacterium that causes severe diarrhoea and inflammation of the colon, leading to a high risk of disease and death. Low complexity in the diversity of species present in the microbiota, a characteristic commonly observed for inhabitants of industrialized nations, is associated with enhanced susceptibility to infectious diseases6. Moreover, mice that have either been treated with antibiotics or raised in germ-free conditions, and thus lack a microbiota, are more susceptible to intestinal pathogens than are mice with a normal microbiota7.
Conversely, certain microbiotas might lead to the promotion of pathogen growth or infection at a higher level of virulence. For example, different mouse microbiotas determine susceptibility to the pathogen Citrobacter rodentium, which causes a type of abnormal growth in the colon called hyperplasia8. Microbiota transplantation from susceptible to non-susceptible mice induces susceptibility to C. rodentium infection, whereas microbiota transplantation from non-susceptible to susceptible animals generates resistance to infection8. Epidemiological evidence indicates that susceptibility to infections by the food-borne pathogen Campylobacter jejuni in people in Sweden depended on the species composition of their microbiota9. And reports have highlighted how certain gut pathogens, such as Salmonella enterica and C. rodentium, exploit host–microbiota cues to precisely modulate their metabolism and, through the process of respiration, their energy generation. By sensing and responding to these cues, pathogens can also increase or decrease expression of components of their virulence repertoire, which are used to colonize the host10–12.
Exciting research is starting to investigate the role of the microbiota in infection. Such work is going beyond just documenting correlations between infection and the presence or absence of species, or differences in species composition. It is beginning to unravel the mechanisms by which particular compositions of microbiota offer resistance to infection or aid invading pathogens.
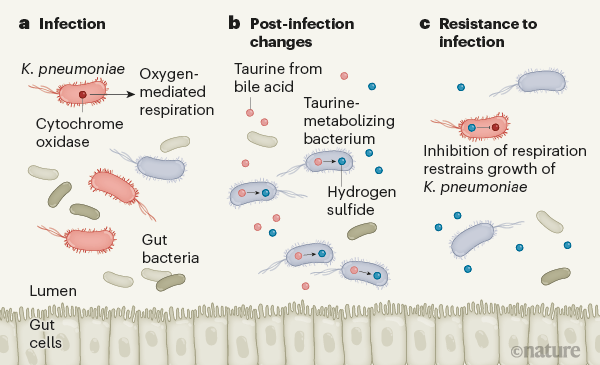
Stacy and colleagues report that, after infection with the gut pathogen Klebsiella pneumoniae, mice have an enhanced ability to resist subsequent infection by this bacterium (Fig. 1). To try to understand the pathway responsible, the authors analysed microbial DNA, assessing the metagenomes (all of the microbial genes detected in the community) from post-infection microbiotas and naive microbiotas (those not previously exposed to the bacterium), to determine how microbes might contribute to colonization resistance. The team found that genes encoding proteins needed for the metabolism of sulfur-containing molecules, including taurine, were much more enriched in the post-infection microbiotas than in the naive microbiotas.
Bile acids are made in the liver and stored in the gall bladder, and they are the main source of taurine in the gut. They are secreted into the intestine to aid the digestion of fatty foods and oils. Specific members of the microbiota break down bile acids, releasing taurine, which can serve as an energy source for other gut bacteria. The use of taurine in bacterial metabolic pathways generates the compound hydrogen sulfide as a by-product. At high concentrations, hydrogen sulfide can inhibit the activity of cytochrome oxidase enzymes, which catalyse reactions that occur during oxygen-dependent (aerobic) respiration.
Invading intestinal pathogens often exploit host-generated oxygen to obtain energy by aerobic respiration and thus gain a foothold in their efforts to colonize the host13. Stacy and colleagues report a correlation between taurine-mediated production of sulfide molecules, including hydrogen sulfide, by the microbiota after infection and the concomitant inhibition of pathogen respiration, which would ultimately inhibit infection by the pathogen. The authors demonstrated this effect for two pathogens, K. pneumoniae and C. rodentium, which suggests that the post-infection microbiota provides broad protection against invaders. Notably, Stacy et al. report that taurine supplementation in the animals’ drinking water led to similar effects. Taurine is a common component of energy drinks, and this discovery of its role in the gut is intriguing. A deeper understanding of such mechanisms could open the door to precision manipulation of the microbiota to combat certain infectious diseases.
These results suggest that dietary supplementation of certain metabolites, such as taurine, might offer a way to reprogram the microbiota’s ‘metametabolism’ to enhance resistance to pathogens. This and other studies defining the mechanisms by which the microbiota affects the metabolism, respiration and virulence of intestinal pathogens represent a key step forwards in the field of host–microbiota–pathogen interactions.